All published articles of this journal are available on ScienceDirect.
A Review on Medicinally Important Heterocyclic Compounds
Abstract
Heterocyclic compounds account for the most prominent and diverse class of organic compounds. A significant number of heterocyclic compounds have been synthesized up to this point. Heterocyclic compounds are rapidly increasing in number due to extensive synthetic research and also their synthetic utility. Such compounds have a wide range of uses in the field of medicinal chemistry. Dyestuff, sanitizers, corrosion inhibitors, antioxidants, and copolymer synthesis are additional well-known applications. There are always distinguishing characteristics of an efficient approach for producing newly discovered heterocyclic compounds and their moieties. According to prior research, more than 90% of medicines containing heterocyclic compounds have been developed after the obtainment of a thorough scientific grasp of the biological system. It was discovered in the neoteric developments of heterocyclic compounds that these play a vital role in curative chemistry, and exert anticancer, anti-inflammatory, antifungal, antiallergic, antibacterial, anti-HIV, antiviral, anti-convulsant, and other biological activities. The present article provides detailed information regarding such heterocyclic compounds.
1. INTRODUCTION
Heterocyclic compounds, often known as heterocycles, are organic chemical compounds having a ring-like structure that includes one or more heteroatoms. Heterocycles can be both cyclic and acyclic [1-14]. The general structure of heterocycles is similar to that of cyclic organic compounds, which have only carbon atom in their structure, but the substitute of one or more carbon atoms by heteroatoms gives heterocycles physico-chemical properties that are distinct from those of all carbon ring analogs [15-17]. Heterocycles involve a wide range of uses, including agrochemical, medicinal, and veterinary [18]. Such compounds are also used in sanitizers, antioxidants, copolymers, corrosion inhibitors, dyestuff [19], etc. Heterocycles are currently employed in the production of a wide range of organic chemical substances [20]. Several compounds, mostly of natural origin, such as alkaloids, morphine, vinblastine, and reserpine, and a variety of antibiotics, such as cephalosporin, penicillin, and others, include heterocyclic components [21].
According to data, heterocycles are present in more than 85% of all physiologically active chemical compounds. This emphasizes the significance of heterocycles in modern drug design [22-32]. All heterocycles, synthetic and natural, exert pharmacological activity [33]. Heterocyclic compounds, which are physiologically and pharmacologically active, have gained prominence in the medicinal study [34]. Many biological compounds associated with living organisms, such as vitamins, hormones, and antibiotics, are composed of heterocyclic molecules [35]. Heterocyclic compounds with nitrogen atoms in their structures are regarded as the major class of chemical substances among physiologically active complexes, natural products, and chemicals widely employed in medicinal chemistry [36, 37]. Among these nitrogen-containing heterocyclic compounds, quinoline, indoles, pyrroles, and pyrrolidines have gained importance in many research sectors, including organic synthesis and medicine. Because of their numerous uses, the production of heterocyclic compounds has become a focal point in organic synthesis [38, 39]. Many systematic methods for producing nitrogen-containing heterocyclic compounds were conceived and developed in earlier decades [40]. In addition to the full-scale research of heterocycles, particularly nitrogen heteroatom-based heterocycles, scientists have demonstrated a great interest in other heterocycles, sulfur-containing heterocyclic molecules [41]. Sulfur-containing heterocyclic compounds make up a large portion of FDA-approved medicines and therapeutically dynamic structures. These chemicals have been proven to exert anti-diabetic, antibacterial, anticancer, antiviral, antimicrobial, anti-inflammatory, anti-hypertension, antimalarial, anti-Alzheimer’s, antifungal, and many other biological activities. Sulfur-containing heterocyclic compounds are widely utilized in chemical research and are found in a variety of natural goods and medicines. Furthermore, numerous sulphur-containing heterocyclic compounds are used to flavor food products, such as meat, vegetables, peanuts, coffee, and cocoa [42-44]. Several FDA-approved medicines include sulfur heterocycles, such as clopidogrel, raloxifene, and rosiglitazone, which are used to treat peripheral arterial disease, breast cancer, and diabetes, respectively [45]. Likewise, ritonavir is a well-known antiviral agent [46]. Thiabendazole can also be used as an antifungal agent. Apart from that, several medicines containing sulfur heterocycles are FDA-approved and are used for a diverse range of medical disorders [47-60].
2. MEDICINAL APPLICATIONS
2.1. Anticancer Activity
Cancer is a collection of diseases distinguished by irregular or uncontrolled cell growth with the ability to occupy or spread to other parts of the body. This disease is caused by a variety of agents, including chemical compounds and radiant energy. Several medications are used to cure this disease, either by killing cancer cells or altering their growth.
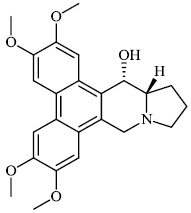
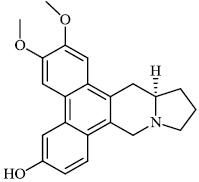
Liu et al. reported the synthesis of phenanthroindolizidine 1 and phenanthroquinolizidine 2 alkaloids for potential use as anticancer drugs with IC50 values of 166 nM and 2.1 nM, respectively. The majority of synthesized compounds exhibited active proliferative action in opposition to BEL-7402 and A549 cells. In the primary screening, compound 2 was discovered to have the most potent activity. A mechanistic analysis revealed that compound 2 potently suppressed cell growth and colony formation, which are associated with a delay in S phase advancement via the inhibition of the DNA synthesis [61].
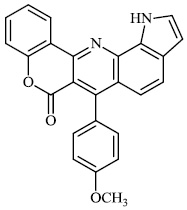
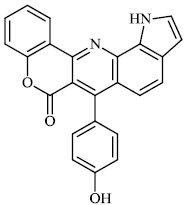
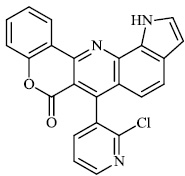
Thigulla et al. described the synthesis of fused chromeno [4, 3-b] pyrrolo [3, 2-h] quinolin-7(1H)-one compounds 3-5 with IC50 values of 228.5 μM, 197.7 μM, and 70.74 μM, respectively. The synthesized compounds were tested for anticancer activity using murine melanoma cell lines (B16F10). To increase their efficacy, their molecules can be further replaced with different substituents (Figs. 1 and 2) [62].
Morsy et al. synthesized a series of coumarin-containing compounds 6-8 with IC50 values of 91.1±5.27 μg/ml, 5.5±0.19 μg/ml, and 52.0±3.55 μg/ml, respectively, and evaluated their activity against human tumor cell lines. The synthesized compounds were the most active against MCF-7 and HepG-2 cell lines (Figs. 3-5) [63].

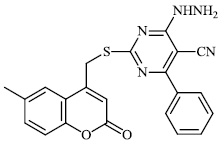
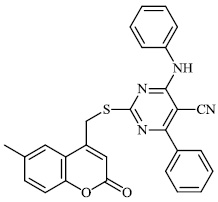
Aboraia et al. explored the synthesis of a series of 5-(2-hydroxyphenyl)-3-substituted-2, 3-dihydro-1, 3, 4-oxadiazole-2-thione derivatives 9 as potent anticancer agents (MCF-7: 32-104). In the primary assay, the synthesized compounds demonstrated high anticancer activity and were selected for a comprehensive anticancer screening in opposition to a 60-cell panel assay, where they demonstrated potential anti-cancer activity (Figs. 6-8) [64].
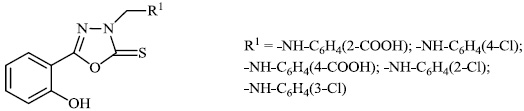
Wang et al. synthesized C-11 labeled fluorinated 2-arylbenzothiazoles 10 (GI50< 0.1 nM for MCF-7 and MDA 468 breast cancer cell lines), which are employed in positron emission tomography (PET) imaging of tyrosine kinase in cancer. Fluorinated 2-arylbenzothiazoles are novel prospective anticancer medicines that inhibit breast, lung, and colon cancer cell lines effectively and selectively (Fig. 9) [65].
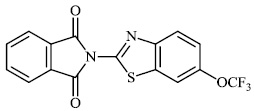
Kok et al. synthesized phthalimide-containing benzothiazole 11 with an IC50 value of 69 μM and tested its anticancer efficacy on human carcinoma cell lines. The authors discovered that the toxicity of synthesized benzothiazole-containing phthalimide on bone marrow cells was comparable to that of cancer cells [with 50% of cellular ATP content loss around 69 μM (25 μg/ml)] (Fig. 10) [66].
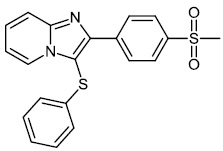
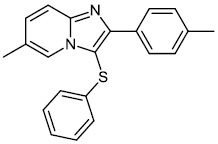
Chitrakar et al. reported the synthesis and anticancer efficacy of sulfenylated 2-phenylimidazo [1, 2-a] pyridines 12-14. All compounds demonstrated good to excellent activity against different human cancer cell lines, i.e., HepG2 (liver), MDA MB 231 (breast), A549 (lung), SKMEL-28 (skin melanoma), Hela (cervical), U87MG (glioblastoma), and DU-145 (prostate) cell lines by employing the MTT assay (Fig. 11) [67].
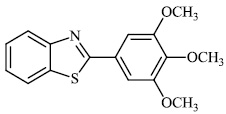
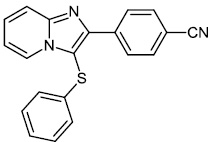
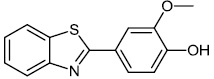
Fu et al. reported the synthesis of 2-(4-hydroxy-3-methoxyphenyl)-benzothiazole. The synthesized compound inhibited breast cancer cell proliferation and invasiveness. Furthermore, compound 15 suppressed the capacity to form spheres and boosted the expression of the carboxyl terminus of Hsp70-interacting protein, which inhibits the oncogenic pathway, and hence, lowers the tumorigenic and metastatic potential of breast cancer cells (Figs. 12-14) [68].
2.2. Anti-Inflammatory Activity
Anti-inflammatory is a term used to describe drugs that are used to relieve or mitigate inflammation or swelling. Analgesics account for about half of all anti-inflammatory medications. In contrast to opioids, which damage the central nervous system and inhibit pain signaling to the brain, NSAIDs relieve pain by reducing inflammation. Aspirin, ibuprofen, and naproxen are the most commonly used anti-inflammatory drugs and are known as Non-steroidal Anti-inflammatory Drugs (NSAIDs); this term distinguishes these drugs from steroids. Though having a common mode of action, the newer specific cyclooxygenase inhibitors are not listed with the conventional NSAIDs. Prolonged use of NSAIDs can result in gastric erosions, and in acute situations, fatal hemorrhage. For adults aged 16-45, the chance of death from NSAIDs use is 1 in 12,000. For those above the age of 75, the chances almost double. Other risks of NSAIDs include asthma exacerbation and kidney injury. In addition to aspirin, pharmaceutical and over-the-counter NSAIDs raise the risks of stroke and myocardial infarction (Fig. 15).
Sondhi et al. reported the synthesis of 2-thiopyrimidine derivatives, for example, 7, 7, 8a-trimethyl-Hexa-hydro-thiazolo [3, 2-c] pyrimidine-5-thione 16. The synthesized compounds were tested for biological activity and showed good analgesic (50%), anti-inflammatory (37.4%), and kinase (CDK-1; IC50: 0 μM, CDK-5; IC50: >10 μM and GSK-3; IC50: >10 μM) inhibitory activities. Carrageenan-induced paw edema in albino rats was used to screen their anti-inflammatory efficacy. Edema in one of the hind paws was generated by injecting 0.1ml of 1% carrageenan solution into the plantar aponeurosis [69].
Perner et al. synthesized 6, 7-disubstituted 4-aminopyrido [2, 3-d] pyrimidine 17 (IC50 (enzyme): 350±50 nM and IC50 (intact cells): 1500±289 nM), and this compound has been stated to be effective in the treatment of inflammation, epilepsy, sepsis, etc. (Fig. 16) [70].
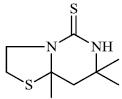
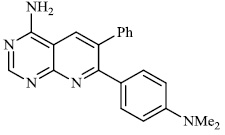
Balkan et al. described the synthesis of some thiazolo [4, 5-d] pyrimidine-7 (6H)-one derivatives and investigated them for different biological activities. Compound 18 (ED50: 129 mg/kg) demonstrated anti-inflammatory and analgesic properties similar to acetylsalicylic acid, while compound 19 showed high anti-inflammatory activity (35%) (Fig. 17) [71].
Shehata et al. synthesized imidazo [2, 1-a] [1, 2, 4] triazolo [1, 5-c] pyrimidine 20 and 1, 2, 4-triazolo [1, 5-c] pyrimido [2, 1-a] pyrimidine 21, and explored them for their potent anti-inflammatory action on carrageenan-induced edema in rat paws (Figs. 18 and 19) [72].
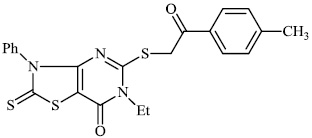
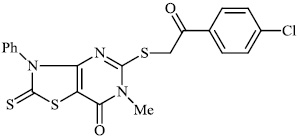
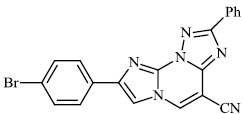

Mochona et al. accomplished the synthesis of tetrahydro pyridine derivatives 22 and 23 with substantial anti-inflammatory activity. The impact of substituents on pharmacological activity was tested in male Sprague-Dawley rats using the carrageenan-induced paw edema experiment. Analogs containing electron-donating substituents at positions 4 and 2 of the benzene moiety displayed strong anti-inflammatory effects, similar to indomethacin (Figs. 20 and 21) [73].
Amir et al. synthesized 2-substituted aryl 1, 3, 4-oxadiazoles 24 and tested them for anti-inflammatory action (22.34% to 72.34%). Several synthesized compounds were found to have anti-inflammatory properties similar to the standard drug ibuprofen. Furthermore, when compared to standard antibiotic ofloxacin, all of these compounds exhibited considerable antibacterial efficacy against S. aureus and E. coli (Figs. 22 and 23) [74].
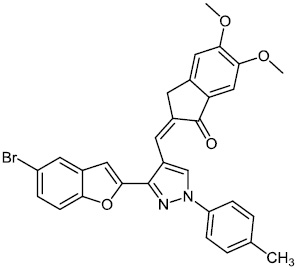
Kanchappa et al. synthesized benzofuran pyrazole heterocycles 25 and screened them for their anti-inflammatory efficacy. The synthesized compounds exhibited the ability to inhibit the edema caused in the rat's hind paw following injection of a phlogistic agent, such as carrageenan (Fig. 24) [75].

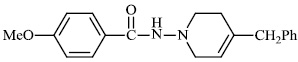
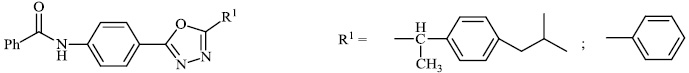
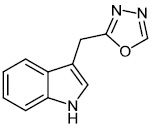
Kumar et al. synthesized indole functionalized oxadiazoles 26 as anti-inflammatory agents. The anti-inflammatory and analgesic efficacy of indole functionalized oxadiazole derivatives was investigated; they displayed anti-inflammatory and analgesic properties equivalent to the reference drugs (Fig. 25) [76].
2.3. Antiviral Activity
A virus is a parasitic organism that cannot replicate on its own. On the other hand, a virus can direct the cell machinery to develop more viruses once it has infected a susceptible cell. The genetic material in most viruses is either RNA or DNA. The nucleic acid and an outer protein shell make up the whole infectious virus particle, known as a virion. The FDA has approved antiviral agents for the treatment of viral infections. Antiviral drugs mainly target different stages of the viral life cycle.
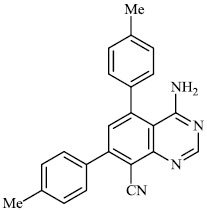
Held et al. reported the synthesis of 4, 5, 7, 8-substituted quinazolines 27-30 (EC50: 0.6±0.1 μM). The synthesized compounds exhibited significant activity against HCMV (Fig. 26) [77].

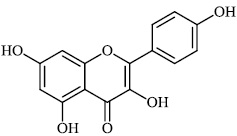
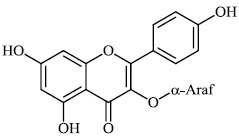
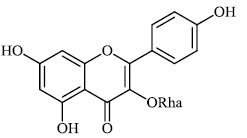
Schwarz et al. reported the synthesis of kaempferol and kaempferol glycosides as good candidates for 3a channel proteins of coronaviruses. The kaempferol compound 31 with an IC50 value of 20 μM could be used to produce novel antiviral agents with increased bioavailability. In particular, the glycosides of kaempferol 32 and 33 with IC50 values of 2.3 μM and 10 μM, respectively, appear to be important candidates for exploration as anti-coronaviral drugs. The significance of multi-target medicines is highlighted by the fact that they block the 3a channel, inhibiting virus replication and obstructing other viral life cycle stages (Figs. 27-30) [78].
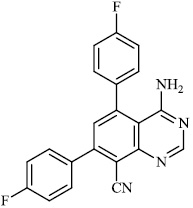
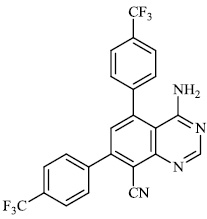
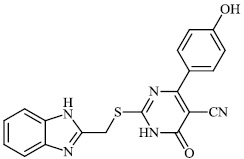
Diwani et al. investigated benzimidazole derivatives 34-36 with IC50 values of 0.6 μM and 1.5 μM, respectively, and screened them for their anti-HCV efficacy. Compound 36 was found to exert significant activity (Figs. 31-34) [79-81].
Hagar et al. studied some nitrogen-containing heterocyclic compounds as inhibitors for Covid-19, including favipiravir 37, amodiaquine 38, 2'-fluoro-2'-deoxycytidine 39, ribavirin 40, hydroxychloroquine 41, and remdesivir 42, with a binding affinity of −4.06, −7.77, −4.47, −4.69, −6.06, and −4.96 kcal/mol, respectively (Figs. 35 and 36) [82].
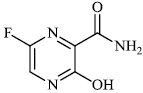
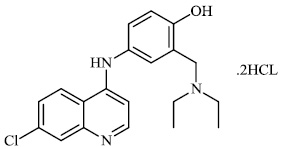
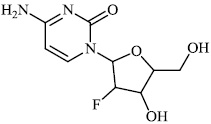
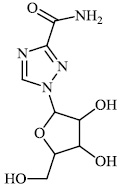
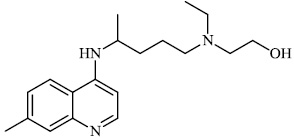
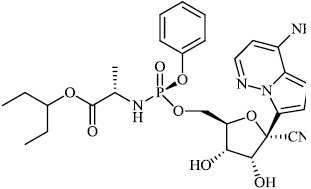
Hwa et al. explored some coumarin-based heterocyclic compounds 43 (CC50: 178μM, 117μM and 144 μM respectively; EC50: 19.1μM, 10.2μM and 17.2 μM respectively) and 44 (CC50: 126μM and 107 μM; EC50: 58μM and 19.0 μM) as the most potent inhibitors against the chikungunya virus (CHIKV) (Figs. 37-42) [83, 84].
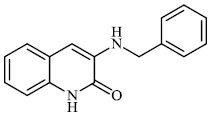
Diaz et al. synthesized and tested quinoline derivatives 45 for antiviral activity. The synthesized quinoline derivatives displayed remarkable antiviral activity (Figs. 43 and 44) [85].
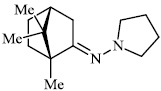
Kovaleva et al. synthesized N-heterocyclic hydrazine derivatives of camphor. Compound 46 showed the highest activity against the H1N1 influenza virus (Figs. 45 and 46) [86].
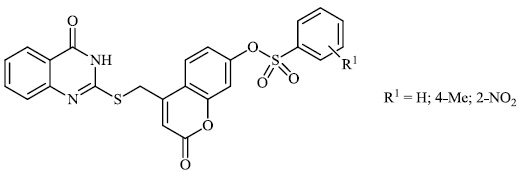
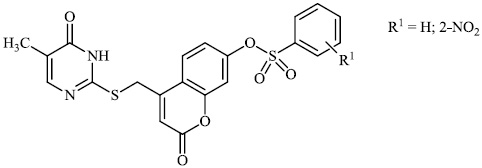
2.4. Antibacterial Activity
Bacteria are single-cell organisms that can be found individually or in groups. Many effective and generally non-toxic medications available to treat bacterial infections pose challenges for medicinal chemists. Antibacterials, often known as antibiotics, are used to prevent or cure bacterial infections by either killing or inhibiting the development of bacteria.
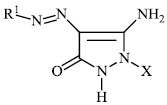
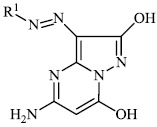
Azab et al. synthesized novel heterocyclic compounds with a sulfonamide moiety, such as aminopyrazole derivatives 47, pyrazolopyrimidine derivative 48, and pyrimidine and thiazine derivatives 49 and 50, and assessed them for their antibacterial efficacy. Most of the synthesized compounds exhibited promising antibacterial properties againstGram-positive and Gram-negative bacteria [87].
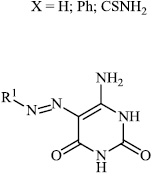
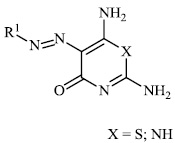
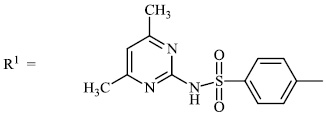
El-Hashash et al. synthesized heterocyclic chalcone derivatives 51, 53, 54, and spiro heterocycles 52 and tested them for their antibacterial activity. The majority of the synthesized compounds showed strongest antibacterial efficacy against all the microorganisms tested with the diameter of the zones equal to 1.1-1.2 cm (moderate activity; 55-65%) and 1.8-2.0 cm (high activity; 85-100%), respectively (Figs. 47-50) [88].
Bouzian et al. synthesized novel quinoline derivatives 55 and 56 and screened them for their potent antibacterial activity against E. coli, S. aureus, S. faecalis, and P. aeruginosa bacterial strains. They showed remarkable antibacterial activity against E. coli and S. aureus with MIC values of 6.25 μg/ml, respectively (Figs. 51-54) [89].
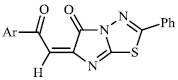
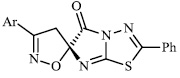
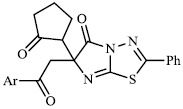
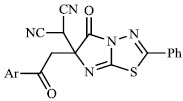

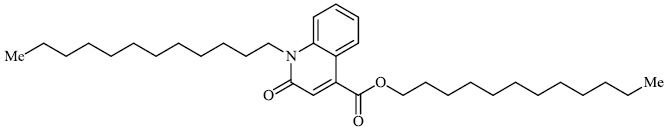
Arshad reported the synthesis of a series of heterocyclic derivatives bearing pyrimidine 57, oxazole 58, and pyrazole 59 nuclei. Compounds were assessed for their antibacterial activity with MIC values of 6.25 and 12.5 μg/ml, respectively. Compound 59 was found to exert the most significant antibacterial efficacy compared to ciprofloxacin (Figs. 55 and 56) [90].
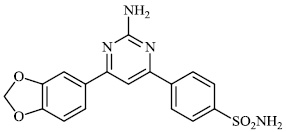
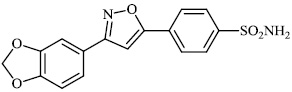
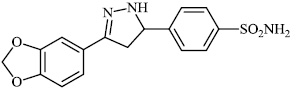
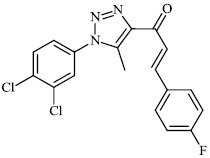
Santosh et al. synthesized novel triazole-linked chalcone derivatives 60 and 61, and screened them for their antibacterial efficacy against B. subtilis, P. aeruginosa, S. aureus, and E. coli with MIC values ranging between 44-79 mM and 63-82 mM, respectively (Figs. 57-59) [91].
Kritchenkov et al. synthesized novel chitosan derivatives 62 and tested them for their antibacterial activity. When compared to commercial antibiotics ampicillin and gentamicin, the synthesized compound exhibited a strong antibacterial activity (Figs. 60 and 61) [92].
Burmeister et al. synthesized Ruthenium (II) N-heterocyclic carbene complexes as antibacterial agents and bacterial thioredoxin reductase inhibitors. Compound 63 was found to be the most potent against S. aureus strains, having an MIC value of 19.5 μM (Figs. 62 and 63) [93].
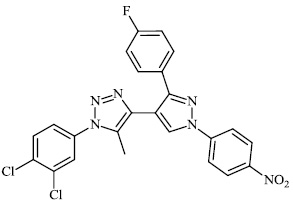
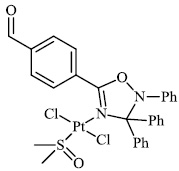
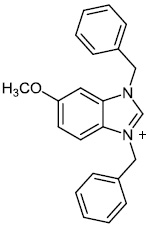
2.5. Anti-Alzheimer’s Activity
Alzheimer's disease (AD) is the most frequent degenerative brain disorder and is characterized by cognitive impairment. Patients with Alzheimer's disease lose their capacity to code new memories, making life incredibly challenging. In this field, new drugs are being developed at a rapid speed.
Osmaniye et al. synthesized a new class of thiazole-piperazine derivatives 64 and 65 with IC50 values as 0.0496±0.002μM, 0.0317±0.001μM, and 0.2158±0.010 μM, respectively, to combat Alzheimer's disease. The acetylcholinesterase (AChE) enzyme was significantly inhibited by all the synthesized compounds. On the other hand, none of the substances inhibited the butyrylcholinesterase (BChE) enzyme significantly [94].
Abdalla et al. synthesized some pyrimidine and thiopyrimidine hybrids 66 and 67 with IC50 values of 4.10 nM, 3.41 nM, 3.28 nM, and 9.51 nM, respectively, fused with steroidal structure and tested them against Alzheimer’s disease. Most of these compounds have shown remarkable activity against Alzheimer's disease (Figs. 64 and 65) [95].
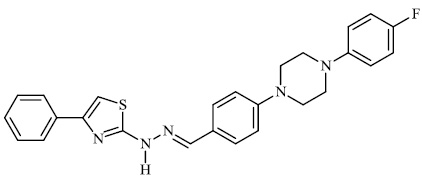

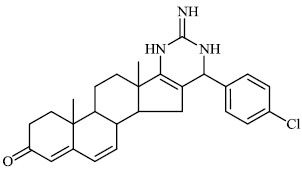
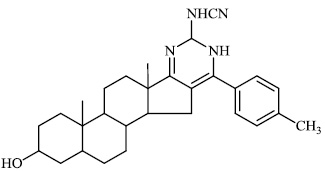
Attaby et al. synthesized bipyridine derivatives 68 and evaluated them for their anti-Alzheimer’s activity. Their relative efficacy as anti-Alzheimer drugs in comparison to flurbiprofen is high enough; however, their relative potency was found to reduce following their reactions to afford the equivalent bipyridine-5-carbonitriles (Figs. 66-68) [96].
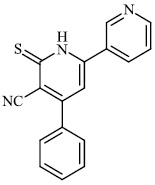
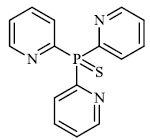
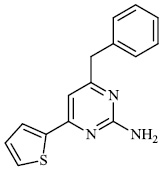
Gulcin et al. synthesized tris (2-pyridyl) phosphine (selenide) sulfide 70, 4-benzyl-6-(thiophen-2- yl) pyrimidin-2-amine 71, and sulfur-containing pyrroles 69 as potential inhibitors against Alzheimer’s disease. The synthesized compounds showed IC50 values in the range of 13.51-26.55 nM against α-glycosidase, 0.54-31.22 nM against BChE, and 13.13-22.21 nM against acetylcholinesterase (AChE). As a result, nitrogen, phosphorus, selenium, and sulfur-containing heterocyclic compounds exhibited significant inhibitory profiles against the identified metabolic enzymes [97].

Attaby et al. synthesized bipyridine derivatives 68 and evaluated them for their anti-Alzheimer’s activity. Their relative efficacy as anti-Alzheimer drugs in comparison to flurbiprofen is high enough; however, their relative potency was found to reduce following their reactions to afford the equivalent bipyridine-5-carbonitriles (Figs. 66 and 67) [96].
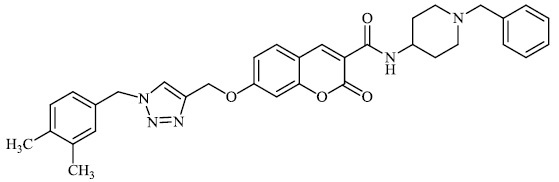
Rastegari et al. synthesized 1, 2, 3-triazole chromenone carboxamide derivatives 72 as potent inhibitors against acetylcholinesterase with an IC50 value of 1.80 μM. Furthermore, this compound demonstrated adequate neuroprotection against H2O2-induced cell death in PC12 neurons, as well as metal chelating Activity toward Fe2+, Cu2+, and Zn2+ ions (Figs. 69-71) [98].
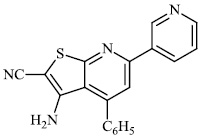
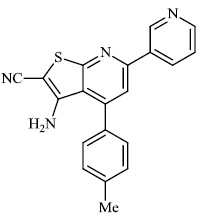
Attaby et al. synthesized 2-substituted thienopyridines and tested their activity against Alzheimer's disease. In general, the compounds with phenyl moiety 73 showed high activity against Alzheimer's disease as compared to the compounds with phenyl-p-methoxy moiety 74. Also, the potency of synthesized compounds as anti-Alzheimer’s agents relative to flurbiprofen is high enough (Figs. 72-74) [99].
Latif et al. reported the synthesis of benzimidazole-2-thiol-based heterocycles and evaluated them for their in vitro anticholinesterase activity. AChE and BChE enzymes were evaluated at various doses ranging from 62.5 to 1,000 μg/ml. Two of the produced compounds, that is, 75 and 76, were shown to be substantially active against the tested enzymes. Compound 75 was discovered to be a dual inhibitor, having IC50 values of 121.2 (AChE) and 38.3 μM, respectively (BChE) (Figs. 75 and 76) [100].
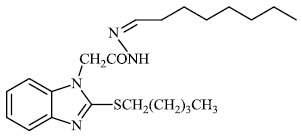
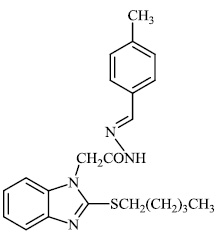

Husain et al. reported the synthesis of coumarin-based heterocyclic hybrids and screened them for their activity against Alzheimer's disease. Compound 77 was found to be the most effective AChE inhibitor (IC50 = 0.091 μM), whereas compound 78 was shown to be extremely active against BuChE (IC50 = 0.559 μM) (Figs. 77-79) [101].
2.6. Antidiabetic Activity
Diabetes Mellitus is a collection of metabolic disorders defined by a persistently high blood sugar level. The most prevalent symptoms of diabetes include increased appetite, increased thirst, and frequent urination. Diabetes, if left untreated, can lead to several health issues. Cardiovascular disease, nerve damage, stroke, cognitive impairment, eye damage, foot ulcers, and chronic renal disease are all serious long-term consequences of diabetes. Diabetes is caused by either a lack of insulin production by the pancreas or the body cells incapable of responding appropriately to the insulin produced. An enormous number of medications are available to treat diabetes mellitus by lowering the blood glucose level. With the exceptions of insulin, exenatide, and pramlintide, all are administered orally and are thus called oral hypoglycemic medications.
Prabhat et al. synthesized benzothiazole derivatives and explored their antidiabetic activity. In diabetic rats, the synthesized compounds 79 caused a greater drop in blood glucose compared to other compounds. The LD50 values of the synthesized compounds were estimated to be in the range of 100-1000 mg/kg, respectively [102].
Nabil et al. synthesized curcumin-based heterocyclic compounds 80 and 81 with IC50 values of 200.2 μM and 95.5 μM, respectively, as potent antidiabetic agents. The authors revealed that pyranone and pyrimidinone derivatives of curcumin exhibited high potential against diabetes (Figs. 80 and 81) [103].
Panahi et al. synthesized novel pyrimidine-fused hybrids 82 and 83 with IC50 values of 148±1 μM and 9±1 μM, respectively, as strong antidiabetic α-glucosidase inhibitors. Both compounds displayed excellent inhibitory activity against yeast α-glucosidase. In addition, compound 82 also exhibited inhibitory activity against mouse α-glucosidase (Figs. 82 and 83) [104].

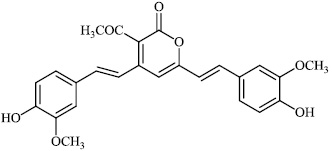
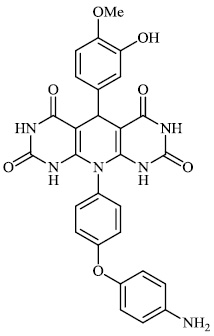
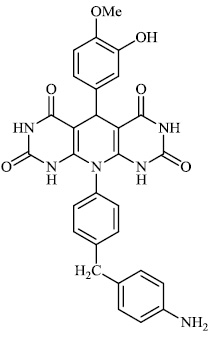
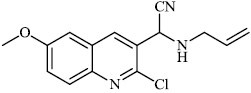
Dalavai et al. synthesized quinolinyl amino nitriles and evaluated them for different biological properties. Compounds 84 and 85 demonstrated promising antidiabetic activity with IC50 value of 100 μg/ml (Figs. 84 and 85) [105].
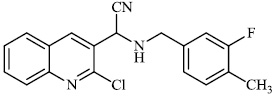
Mamatha et al. synthesized mercaptooxadiazole derivatives 86 as antidiabetic agents with 62% inhibitory potency and anti-tubercular agents with an MIC value of 1.6 μg/ml. Compounds with benzoyl, p-chlorobenzoyl, heptyl, and p-methylbenzoyl substituents displayed moderate activity against diabetes (Fig. 86) [106].
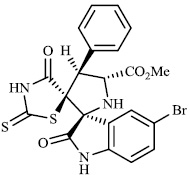
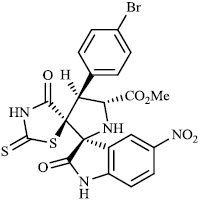
Toumi et al. synthesized rhodanine-fused spirooxindole pyrrolidine hybrids 87 with IC50 values of 1.49±0.10, 1.50±0.07, and 1.57±0.10 μM±SD, respectively, and 88 with IC50 value of 1.59±0.08 μM±SD as new α-amylase inhibitors. The majority of the synthesized compounds demonstrated high α-amylase inhibition (Figs. 87 and 88) [107].

2.7. Antifungal Activity
Fungi are organisms that do not belong to animal or plant kingdoms. They can be found in the soil, wet places, air, plants, water, decaying organic materials, as well as in animals and humans. Fungi, together with bacteria, perform a crucial function in our environment by reducing organic matter into simpler forms for plant use. They include mushrooms, household yeast, molds, and many others. Aspergillus, Mucormycetes, Histoplasma, Candida, Pneumocystis, Cryptococcus are the most prevalent forms of fungi. In general, several forms of fungi do not cause infections in humans, but opportunistic infections, which affect persons with impaired immunity, can cause illness. Diabetes, blood malignancies, iron overdose, trauma, steroids medication, malnourishment, etc., are some conditions that reduce our immunity.
Mucormycosis, commonly called black fungus, is a deadly but uncommon fungal illness caused by micromycetes, a kind of mold. Few subgroups are typically involved in the occurrence of this infection, including Rhizomucor, Mucor, and Rhizopus. These fungi are angioinvasive, i.e., they enter and damage the surrounding blood vessels, causing tissue necrosis and death. These infections are extremely deadly, and most people would die if they are not treated. Its associated death rate varies between 25% and 90%. Once the infection has migrated to the brain, the risk of death is quite high. As a result, early detection and treatment are given a high priority.
Pain, redness around the nose and/or eyes, coughing, fever, chest pain, shortness of breath, headache, disrupted mental status, bloody vomits, toothache, double vision, loosened teeth with discomfort are all symptoms of black fungus infection.
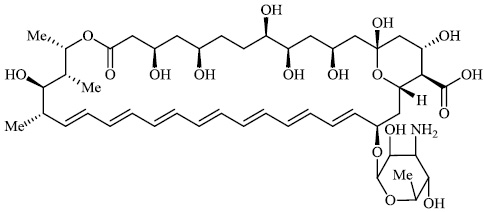
At least 45,432 such cases have been reported in India, mostly among COVID-19 patients as of 15 July 2021. Russia and Pakistan are also witnessing a surge in black fungus infections. According to a senior neurosurgeon at AIIMS, patients who undergoing COVID therapy are at the greatest risk of developing black fungus within six weeks. As the number of black fungus infections rises, the need for the anti-fungal medicine Ampho B 89 also increases [108-116], which is often used to treat the condition (Fig. 89).
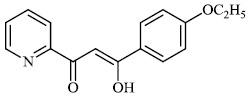
Tighadouini et al. synthesized β-keto-enol pyridine and furan hybrids and screened them for their antifungal activity against Gram-positive strains (B. subtilis and M. luteus) and F. oxysporum. Compound 90 with an IC50 value of 12.83 μg mL−1 demonstrated excellent antifungal efficacy against the tested fungal strains (Fig. 90) [117].
Morcoss et al. synthesized benzimidazole derivatives 91 as strong antifungal agents against C. neoformans var. grubii and C. albicans with MIC values ranging from 4 to 16 μg/ml, respectively (Fig. 91) [118].
Zhao et al. synthesized aromatic heterocyclic derivatives 92 and 93 and studied them for their antifungal activity against C. neoformans, A. fumigatus, and C. albicans strains with MIC values of 0.5, 4, and 0.0625 μg/ml, respectively (Figs. 92 and 93) [119].

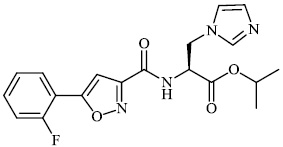
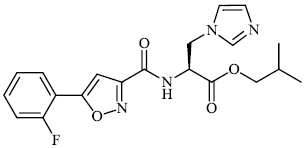
Mi et al. synthesized chitosan-based heterocyclic derivatives 94 with enhanced antifungal action against two plant pathogenic fungi (P. asparagi and C. lagenarium). All compounds displayed strong antifungal activity. For example, the inhibitory indices of 2-fluoroaniline-carboxymethyl chitosan conjugates (FANCMCS) were estimated as 46.24%, 84.35%, and 94.73%, when the series of concentrations were 0.1 mg/mL, 0.5 mg/ml, and 1.0 mg/ml, respectively (Fig. 94) [120].
Chandrika et al. synthesized alkylated mono-, bis-, and trisbenzimidazole derivatives and tested them against fungal strains. Compared to the clinically effective antifungal agents POS (posaconazole), VOR (voriconazole), AmB, and ITC (itraconazole), the synthesized compounds were more effective against certain strains. Compounds 95-97 demonstrated strong activity against C. albicans ATCC MYA-2310(S), C. albicans ATCC 64124, and C. albicans ATCC 90819 (R). Also, compound 95 showed equal or good activity than AmB against A. nidulans ATCC 38163, C. glabrata ATCC 2001, and C. parapsilosis ATCC 22019 with MIC values ranging from 15.6-0.975 μg/ml, respectively (Figs. 95-97) [121].
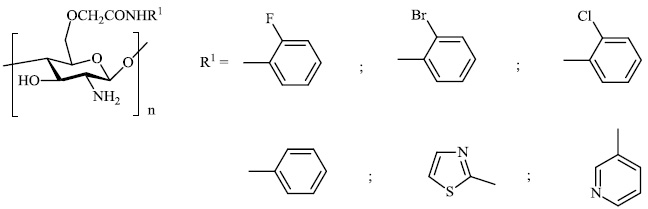
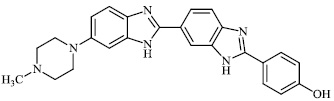

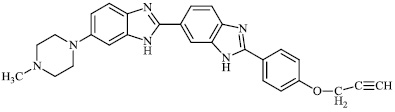
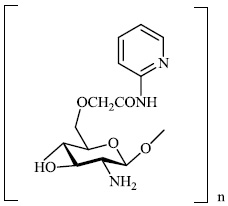
Mi et al. reported the synthesis of chitosan derivatives 98 bearing heterocyclic moieties with remarkable antifungal activity. In vitro antifungal efficacy against two plant pathogenic fungi (Colletotrichum lagenarium and Phomopsis asparagi) was determined using the plate growth rate technique (Fig. 98) [122].
CONCLUSION
Heterocyclic compounds are one of the most significant types of organic molecules in medicinal chemistry and they are used as medications for various diseases. Numerous impressive accomplishments have shown that heterocyclic compounds have a wide range of therapeutic drug applications. Heterocyclic compounds are versatile synthetic targets and key structural units in organic synthesis and medicinal chemistry because of their exciting biological activities. The potential applications of heterocycles as anticancer, anti-inflammatory, antifungal, antibacterial, anti-Alzheimer's, antiviral, antidiabetic agents, etc., have attracted substantial interest within the pharmaceutical community. Interestingly, an increasing number of heterocycles have been identified as potential drug candidates in ongoing drug development.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Parveen Kumar Sharma is the Editorial Board Member of The Open Medicinal Chemistry Journal.
ACKNOWLEDGEMENTS
Declare none.


