All published articles of this journal are available on ScienceDirect.
The Double Sides of Curcumin and its Therapeutic Prospects
Abstract
Plants have been used for centuries as a treatment for various medical conditions, with over 80% of the population relying on them for healthcare. Curcumin, an aromatic spice from Curcuma longa L., is a significant contributor to this list. Curcumin is non-toxic and has numerous benefits, including anti-inflammatory, antiseptic, antioxidant, and analgesic properties. It contains a high number of antioxidants, which can help treat various ailments, including digestion, smallpox, skin cancer, wound healing, body weight, neurological illnesses, cardiovascular diseases, erectile dysfunction, malaria, chicken pox, urinary tract infections, conjunctivitis, rheumatoid arthritis, chronic anterior uveitis, and liver ailments. Curcumin is also used to enhance overall energy, eliminate worms, regulate menstruation, and address digestive disorders. Curcumin is a versatile pharmacological compound with potent curative and regulated chemo-biological properties, making it effective in addressing various human health conditions. However, it can also have toxic effects. Due to its poor bioavailability, it has slow absorption, fast metabolism, and obligatory elimination. To enhance curcumin bioavailability, drugs that inhibit the curcumin metabolic pathway have been used. This review provides a comprehensive overview of the diverse medicinal benefits of curcumin along with its toxic effects.
1. BACKGROUND
For centuries, plants have been widely employed as a prevalent treatment for many medical conditions in different regions of the world. Moreover, they function as the immediate source of medical treatment in rural regions of many nations, with more than 80% of the populace relying on it as their main healthcare [1].
Turmeric, scientifically known as Curcuma longa L., is cultivated in places that have both tropical and subtropical climates [2, 3]. It is an exceptional aromatic spice with a yellow-orange colour that is obtained from the plant rhizome. The plant thrives on a succulent rhizome or subterranean stem and has a 3-foot-tall height with lanceolate foliage and yellow inflorescences [4, 5]. Curcumin, desmethoxycurcumin (DMC), bisdemethoxy- curcumin (BDMC), and cyclic curcumin are categorized as “curcuminoid” substances. Curcumin is the most significant contributor, whereas cyclic curcumin is the least significant. C. longa contains as part of its chemical composition over 3% curcumin, 1.4% DMC, and 1.2% BDMC [1, 5]. Curcumin, specifically known as 1,7-bis(4-hydroxy-3methoxyphenyl)-1,6-heptadiene-3,5-dione, Fig. (1) possesses a lipophilic property, making it incapable of dissolving in water and ether. Curcumin exhibits stability under the acidic conditions of the stomach. The chemical exhibits tautomerism, with the enolic form being present in organic solvents and the keto form being present in water [6]. Curcumin is obtainable in many formulations, such as capsules, pills, and ointments. Curcumin has received the status of “Generally Recognized as Safe” (GRAS) from the US Food and Drug Administration (FDA) [7].
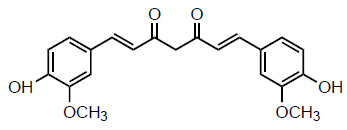
Structure of curcumin.
Curcumin has been utilized for many years due to its variety of pharmacological activities, such as anti-inflammatory, anti-septic, antioxidant, and analgesic properties [6, 7]. Curcumin, as an antioxidant, significantly enhances the treatment or control of digestion, smallpox, skin cancer, wound healing, body weight, neurological illnesses, cardiovascular diseases, erectile dysfunction, malaria, chicken pox, urinary tract infections, conjunctivitis, rheumatoid arthritis, chronic anterior uveitis, and liver ailments [3, 5]. It is also used to enhance overall energy, eliminate worms, regulate menstruation, and address digestive disorders, among other ailments. Researchers have discovered that the application of curcumin ointment to the stomach can effectively penetrate the skin and enhance blood circulation to the genitals [6]. Curcumin also showed promising therapeutic options for the treatment of COVID-19, caused by the Coronavirus [8]. Curcumin is renowned for its potential health advantages and is utilized in many formulations worldwide. Presently, 246 clinical investigations have been reported, from which 115 were successfully completed, employing turmeric components, including curcumin, as therapeutic or dietary supplements for a range of disorders like cancer, type 2 diabetes, cardiovascular diseases, rheumatoid arthritis, chronic kidney diseases, periodontitis, Crohn’s diseases, depression, gynecological diseases, and Alzheimer’s diseases [2-5].
Curcumin is utilized in various ways, such as pharmaceutical supplements, hair ointments, energy drinks, and bathing soaps [6, 8]. Curcumin is a versatile pharmacological compound that possesses potent curative and regulated chemo-biological properties, making it effective in addressing various human health conditions [8]. However, it can also have toxic effects and negative consequence. Nanotechnology potentially addresses some of the shortcomings associated with conventional medicinal treatments with curcumin, particularly low bioavailability, and molecular stability, hence generating considerable scientific interest. The encapsulated curcumin in nanocarriers offers enhanced water solubility, increased penetration via the skin, controlled release, fewer doses, and fewer side effects, among other benefits [9]. This review aims to explore both the positive and negative effects of curcumin, to maximize its prospects for therapeutic use.
2.1. Curcumin: Chemistry, Pharmacokinetics and Bioavailability
2.1.1. Chemistry
Curcumin (diferuloylmethane) is a symmetrical molecule with a molecular mass of 368.385g/mole and a chemical formula of C21H20O6 [10]. The biological activity of curcumin is linked to several significant chemical events, including hydrogen-atom donation reactions that result in its oxidation, enzymatic reactions, hydrolysis, and degradation, as well as reversible and irreversible nucleophilic addition reactions [10]. Curcumin plays a crucial part in various biological activities. Collectively, curcumin can function as a hydrophobic reducing agent, act as an antioxidant, and effectively eliminate different reactive oxygen species (ROS) [11]. As an antioxidant agent, curcumin is reported to be more effective than vitamin E in reducing oxidative stress. Water-soluble antioxidants such as Vitamin C regenerate phenoxyl radicals, so restoring curcumin for further elimination reactions of reactive oxygen species (ROS) [11, 12]. Curcumin exhibits comparable efficacy to endogenous and lipid-soluble antioxidants in enhancing the activity of superoxide dismutase and eliminating superoxide radicals [11].
2.1.2. Pharmacokinetics and Pharmacodynamics
Previous research has addressed the challenge of attaining optimal therapeutic levels of the chemical curcumin because of its limited solubility and inadequate bioavailability [13]. Research indicates that curcumin undergoes biotransformation to di-hydrocurcumin and tetra-hydrocurcumin, and then is converted into mono-glucuronide conjugates [14]. Initial in vivo investigations indicated that curcumin undergoes fast metabolism and conjugation in the liver, followed by excretion in feces with poor systemic bioavailability [15]. Curcumin (40 mg/kg) administration through intravenous route in rats led to total elimination of the substance from the bloodstream within one hour after administration [16]. Administering rats with an oral dosage of 500 mg/kg led to a maximum plasma concentration of barely 1.8 ng/mL [17]. An effective approach to enhance curcumin bioavailability involves the use of drugs that inhibit the curcumin metabolic pathway. A report investigating ways to enhance the absorption of curcumin discovered that intake of curcumin with piperine (from Piper nigrum and Piper longa) increased the levels of curcumin in the bloodstream of rodents [18]. The mechanism involves the inhibition of glucuronidation in the liver and intestine [18]. The administration of high dosages of curcumin (2000 mg/kg) along with piperine resulted in a significant increase in systemic bioavailability, reaching up to a 154% increase [19, 20].
Multiple phase I clinical trials focusing on cancer patients have presented findings on curcumin’s pharma- cokinetics, metabolites, and systemic bioavailability [21-23]. Clinical research was done on 25 patients with different pre-cancerous lesions [24]. These patients were treated daily for 3 months with oral doses of 4, 6, and 8 grams of curcumin. The trial found that the serum curcumin concentrations achieved were only 0.51 ± 0.11, 0.63 ± 0.06, and 1.77 ± 1.87 millimolar (mM), respectively. Nevertheless, safety and patient tolerance were noted even after administering 8 g of curcumin [24]. The concentration of curcumin in the blood reached its highest point within one to two hours after taking the dose and then decreased quickly. No traces of curcumin were found in the urine. Theracurmin, a synthetic nano-particle version of curcumin, was developed to address the issue of low bioavailability associated with curcumin. This new form of curcumin has a greater bioavailability [14].
Dihydrocurcumin (DHC): The role of DHC has been poorly studied. The impact of DHC has been examined in vitro using a human liver cancer cell culture to evaluate non-alcoholic fatty liver disorders (NAFLD) [25]. The features of NAFLD include an increase in oxidative stress, resistance to insulin, inflammation, and hyperlipidemia. DHC increased Nrf2 to mitigate oxidative stress and could potentially reduce insulin resistance by enhancing glucose absorption via positive regulation of the PI3K/AKT pathway. Furthermore, DHC suppresses lipid biosynthesis and enhances lipid oxidation in both HepG2 and L02 cells [25]. DHC was predicted to have a greater binding affinity than curcumin to the active domain of Phospholipase A2 in molecular docking research, indicating the anti-inflammatory properties of DHC [26].
Tetrahydrocurcumin: Tetrahydrocurcumin (THC) exhibits greater stability under physiological conditions and enhanced solubility in aqueous environments compared to curcumin [27]. Conversely, curcumin demonstrates better solubility in lipids and is absorbed more efficiently through the gut while maintaining good stability in plasma. Thus, THC is regarded as a more bioavailable form of curcumin in vivo. As a result, differential administration and metabolism of curcumin and tetrahydrocurcumin in cells may influence their physiological roles [27]. THC had comparable functional activity to curcumin. Numerous in vitro and in vivo investigations have shown that curcumin has superior therapeutic benefits compared to THC, including antioxidant, anti-inflammatory, anti-cancer, and anti-viral effects [28]. Nonetheless, there are recognized functional distinctions between curcumin and THC attributable to their structural differences. In contrast with curcumin, THC does not possess α, β dienes, which accounts for its failure to form Michael adducts with intracellular proteins. Curcumin may interfere with the formation of a disulfide bond by electrophilic dienone. Free thiols on cysteine-rich proteins were reactive with Michael acceptors of curcumin but not with THC [28].
Hexahydrocurcumin: Hexahydrocurcumin (HHC) is a hydrogenated metabolite of curcumin that has received not much attention. It has antioxidant, anticancer, cytotoxic, anti-inflammatory, anti-helminthic, and cardio- protective properties akin to its parent chemical curcumin; in some instances, it demonstrated greater potency. HHC has superior antioxidant activity compared to curcumin due to its enhanced free radical scavenging capabilities [29]. Moreover, in instances of lipid peroxidation and red blood cell hemolysis, it demonstrated superior antioxidant activity compared to curcumin [30]. Research indicated a correlation between structural chemistry and the oxidation resistance of HHC, with an increased amount of hydroxyl groups and phenyl moieties potentially contributing to its enhanced antioxidant properties [30]. The anticancer efficacy of HHC has been examined in the HT29 human colon adenocarcinoma cell line. It suppressed the proliferation of HT29 cells by downregulating COX-2 expression [31].
2.1.3. Bioavailability
Bioavailability is the term used to describe a drug's capacity to be consumed, processed, and excreted by the body. The causes of low curcumin bioavailability were because of slow absorption, fast metabolism, and obligatory elimination [32]. According to several studies, curcumin solutions exhibit lower activity compared to curcumin. However, compared to curcumin alone, its metabolites are more active [33, 34]. The Blood-Brain Barrier (BBB) acts as a protective barrier that restricts the entry of foreign chemicals into the brain [32]. Curcumin exhibits poor solubility in water, and its capacity to penetrate the BBB and reach the brain is attributed to its hydrophobic nature, as well as its nano-formulation [35]. In the present context, nanotechnology-based delivery mechanisms are being utilized to enhance its bio- availability. To resolve the therapeutic challenge of curcumin, one can enhance nanotechnology-based delivery systems to improve circulation and metabolic resistance [35].
2.1.4. Drug-Delivery System
Curcumin has been explored in nanoparticle-based delivery systems that are mostly utilized for hydrophobic medicines with low solubility in water [36]. A separate showed the utilization of a polymer-based nanoparticle called “nano curcumin” with a size of less than 100 nm [37]. The in vitro activity of the compound was discovered to be comparable to that of free curcumin in pancreatic cell lines. A study conducted on healthy volunteers demonstrated the enhanced efficacy of a cream with curcuminoid-loaded solid lipid nanoparticles (SLNs) compared to a cream with free curcuminoids [38, 39]. Overall, more progress is needed in the development of nanoparticle-based methods for curcumin delivery [36].
Positive effects of curcumin.
3. THE DOUBLE SIDES OF CURCUMIN
3.1. Positive Effects of Curcumin
Curcumin has been demonstrated to selectively affect multiple signalling pathways at the molecular level and exhibit cellular activity, suggesting its potential for various health effects [8]. It helps to manage inflammatory and progressive eye conditions, as well as metabolic syndrome, pain, and inflammatory and metabolic syndrome. The consumption of curcumin has numerous health advantages, primarily credited to its antioxidant and anti-inflammatory characteristics (Fig. 2) [40].
3.1.1. Curcumin and Antioxidant Effect
The antioxidant activity of curcumin is a primary mechanism that accounts for most of its effects on different diseases. Research has shown that it enhances the level of antioxidant enzymes specifically superoxide dismutase (SOD), in the bloodstream [41]. Curcumin can counteract free radicals and a range of processes control this. It possesses the capacity to scavenge various types of free radicals, such as ROS and RNS, regulate the function of enzymes that neutralize free radicals, and hinder the activity of enzymes that produce ROS, such as xanthine oxidase/hydrogenase and cyclooxygenase/lipoxygenase [42]. Curcumin is a lipophilic compound that acts as an anti-inflammatory agent by scavenging peroxyl radicals. Curcumin, like vitamin E, is recognized as a chain-breaking antioxidant [42]. Curcumin can attach itself to iron (Fe), manganese (Mn), and copper (Cu), which has been found to affect its antioxidant qualities [43].
Curcumin supplementation at a dosage of 2000 mg per day in postmenopausal women enhanced the immediate decrease in forearm blood flow response to acetylcholine infusions (FBFACh), which is an indicator of resistance artery endothelial function. This effect was observed when the NO synthase inhibitor NG monomethyl-L-arginine was administered [44]. Additionally, curcumin supplementation reduced the immediate increase in FBFACh caused by the antioxidant vitamin C. The study found that it improved the dilation of the brachial artery, which is responsible for the function of the endothelium in the conduit artery. It achieved this by increasing the availability of nitric oxide in the blood vessels and reducing oxidative stress. Additionally, it also improved the function of the endothelium in the conduit artery [44].
In a study involving rats exposed to hepatotoxic levels of mercury (Hg) at a dose of 2.4 mg/kg, pretreatment with curcumin at a dose of 100 mg/kg/day resulted in significant reductions in serum lactate dehydrogenase activity, apoptosis levels, ROS formation, non-protein sulfhydryl levels, total Hg levels, MDA levels, and alanine transaminase activity [45]. Additionally, curcumin pretreatment led to an increase in SOD activity and glutathione peroxidase activity. In addition, it also inhibited the activation of the NF-E2-related factor 2-antioxidant response element (Nrf2-ARE) signalling pathway, leading to a significant increase in the expression of Nrf2, γ-glutamyl cysteine synthetase heavy subunit and heme oxygenase-1 [46].
3.1.2. Curcumin and Anti-inflammatory Effect
Inflammatory cells near the site of inflammation are responsible for the release of several reactive species, leading to oxidative stress. This establishes a clear link between oxidative stress and inflammation [47]. ROS and RNS frequently initiate an intracellular signalling cascade that enhances pro-inflammatory gene synthesis. There is a correlation between inflammation and several long-term diseases and ailments [3, 5, 6].
Curcumin has powerful anti-inflammatory properties, and numerous clinical trials reported its bioactive effects in different inflammatory disorders [48, 49]. Arthritis, a chronic disease, is extensively studied due to its high prevalence. It is characterized by inflammation of the joints, leading to joint damage and dysfunction [50]. The development of this disease has been closely associated with the dysregulation of inflammatory cytokines (tumour necrosis factor, interleukin-1, and interleukin-6), as well as inflammatory enzymes (5-lipoxygenase, cyclooxygenase-2, and matrix metalloproteinase-9) and chemokines [47]. The majority of clinical investigations on the anti-inflammatory effectiveness of curcumin focused on patients with osteoarthritis (OA) and rheumatoid arthritis (RA) [48, 49]. The therapeutic outcomes following the ingestion of curcumin orally were observed by administering various doses (ranging from 200 to 2000 mg/day), different formulations, and different durations of administration (ranging from 2 weeks to 6 months). These effects were assessed using specific symptom scales and/or by measuring inflammatory and stress markers. In their initial clinical investigation, curcumin had a similar impact to phenylbutazone on joint swelling, morning stiffness, and walking time in patients with RA [49].
A separate investigation conducted to validate the anti-inflammatory effects of consuming curcumin orally in patients with type II Diabetes Mellitus (T2DM) revealed a notable enhancement in IL-6, endothelial function, MDA, and TNF-alpha levels in the group treated with curcumin [51]. A study found that curcumin administration resulted in a notable decrease in blood cytokine levels (TGF-b, MCP-1, TNF-a, and IL-6) in patients with metabolic disorders [52]. Therefore, these findings provide robust evidence for the use of curcumin in treating a range of inflammatory disorders.
3.1.3. Curcumin and Skin Diseases
Several reports have shown that curcumin is effective when administered topically for psoriasis, pruritis, dermatitis, and vitiligo [6, 53]. Repigmentation was much better in the group of vitiligo patients who received topical curcuminoid therapy along with the usual narrowband UVB (NB-UVB) treatment [53]. Some research examined the effects of oral curcumin supplementation with psoriasis, a skin illness characterized by chronic inflammation and hyperproliferation, either as a tonic formulation or an ointment [54, 55].
3.1.4. Neuroprotective Activities
One major issue that has prevented curcumin from being used more frequently by people is its low bioavailability [42]. The challenge has been partially or fully addressed by the development of curcumin analogues and derivatives, conjugated curcumin with PLGA, and curcumin encapsulated in liposomes. Analogs and derivatives of curcumin have been used in the course of treatment [42, 56].
The inhibitory role of synaptic proteins, increase in biogenesis, reduction of fission machinery, and mitochondrial fusion activity enhanced curcumin neuro- protective activities against human neuroblastoma (SHSY5Y) cell lines [57]. Multiple studies by various researchers found that supplementing with curcumin (50, 100, and 200 mg/kg) significantly reduced cognitive function, as well as enhanced the mitochondrial complex II and III enzyme activities and antioxidant enzyme activity [58, 59].
Curcumin reduced elevated levels of the acetylcholinesterase enzyme. Researchers found that curcumin protected rats from 6-hydroxydopamine (6-OHDA)-induced neurodegenerative diseases, decreased intercalatum heat shock protein 70 (HSP70), improved their memory, increased levels of acetylcholine (ACh) and dopamine (DA), increased antioxidant enzyme concentrations, decreased levels of monohydroxy- dopamine, and upregulated the expressions of nerve growth factor (NGF), in a rat model of Parkinson's disease [59, 60].
Curcumin therapy attenuated ROS and apoptotic cell death caused by paraquat [61]. Additionally, it increased the expression of genes that protect neurons from damage by reducing oxidative stress and promoting cell death. Gene expression and amyloid precursor protein were both markedly reduced by curcumin pretreatment [61]. Recent research has shown that new curcumin formulations, such as Longvida® and Theracurmin, have great acute and chronic activity [62]. These formulations were improved to ensure better bioavailability, even when administered at significantly lower doses (80–180 mg/day).
3.1.5. Curcumin and Liver Diseases
Curcumin's hepatoprotective properties in people with tuberculosis to see whether it may reduce the risk of liver damage caused by anti-tuberculosis medication have been studied [63]. Curcumin showed encouraging results in individuals with NAFLD, a chronic liver disease defined by the buildup of neutral lipids in liver cells. A study found that individuals with non-alcoholic fatty liver disease (NAFLD) who received curcumin supplements had an improvement in their lipid profile status, body weight and BMI as well as a considerable decrease in AST and ALT (aspartate transaminase and alanine transaminase) levels [64]. Furthermore, supplementing neonatal C57BL/6J male mice with an oral dose of curcumin (100 mg/kg) daily improved histopathological changes in streptozotocin-induced non-alcoholic steatohepatitis (NASH)-hepa- tocellular carcinoma (HCC) by reducing levels of steatosis, fibrosis, and serum aminotransferases. The production of pro-inflammatory cytokines, oxidative stress, and chemokines is significantly reduced in the livers of NASH animals. Not only that, but it also dramatically reduced HMGB1 cytoplasmic translocation and toll-like receptor 4 protein expression [65].
3.1.6. Curcumin and Diabetes
It is widely recognized that T2DM is characterized by an inadequate response of the body to insulin. Since oxidative stress and the production of inflammatory cytokines are closely linked to diabetes, curcumin, which has anti-inflammatory and antioxidant properties, might be a useful treatment option [3, 8]. At 1500 mg daily, curcumin enhanced the activities of beta cells in prediabetic individuals and, most importantly, prevented the development of T2DM in the curcumin group [66]. Supplementation with curcumin reduced insulin resistance index (HOMAIR), hemoglobin A1c test (HbA1c), total serum free fatty acids (FFAs), fasting blood glucose (FBG), and triglyceride (TG) in type 2 diabetics while increasing lipoprotein lipase (LPL) activity, according to another study [67, 68]. Patients who received oral curcumin in addition to normal metformin therapy had a drop in FBG, as well as increased antioxidant status and anti-inflammatory markers. It has been discovered that curcumin is a great supplement to use with glyburide for type 2 diabetes because it increases the bioavailability of the drug by inhibiting permeability glycoprotein (P-gp) [69]. Patients with diabetes who are on glyburide may benefit greatly from curcumin, as it improved their lipid profile and blood glucose levels following a 10-day treatment in this trial.
Intestinal epithelial cells directly bind GLUT proteins, which further reduce dietary glucose absorption, and curcumin has a hypoglycemic impact [70]. Following daily administration of 100 mg/kg of curcumin to Otsuka-Long-Evans-Tokushima Fatty (OLETF) rats, some adverse effects were observed, including albuminuria, ectopic lipid accumulation through activation of AMPK signalling, urinary malondialdehyde, pathophysiologic glomerulus changes, elevated oxidative stress-related Nrf2 signalling, and serum lipid-related index [71].
3.1.7. Curcumin and Its Toxin Neutralizing Effects
Research on the effectiveness of curcumin against genotoxicity following arsenic exposure was stimulated [72, 73]. Reduced DNA damage in lymphocytes, ROS, and lipid peroxidation levels were seen in volunteers exposed to groundwater arsenic who received 1000 mg of curcumin daily [72]. Using a cohort of people exposed to arsenic in West Bengal, a study investigated the effects of curcumin against oxidative stress and the induction of enzymes that repair damage [73]. The study found that curcumin reduced levels of 8-hydroxy-20-deoxyguanosine and the expression of OGG1, a protein crucial for DNA demethylation caused by oxidative stress, while the expression of DNA repair enzymes was increased [73]. After consuming 30 g of curcumin (Theracurmin) with ethanol, a study measured blood ethanol and blood acetaldehyde levels 30, 60, 120, and 180 minutes later [74]. Curcumin users had much lower plasma acetaldehyde concentrations than mineral water drinkers [74]. When it came to ethanol, the outcomes for both groups were rather close. In view of the above, curcumin shows great promise as a means of lowering alcohol consumption.
3.1.8. Curcumin and Cancer Treatment
Several randomized controlled studies in cancer patients have shown the efficacy of curcumin. It alleviates the symptoms and decreases the tumour markers and other characteristics of several malignancies [5, 75, 76]. Oral submucosal fibrosis, oral leucoplakia, and oral submucosal fibrosis in precancerous and cancerous skin lesions were treated with curcumin ointment or pills [77]. Oral curcumin therapy (1–12 g/day) in myeloma patients downregulated COX-2, STAT3, and NF-ƙB expressions and decreased the levels of urine N-telopeptide of type I collagen and paraprotein [75, 78]. Curcumin also showed favourable benefits in individuals with lung, breast, head and neck squamous carcinoma, and prostate malignancies, where tumour markers, secreted mutagens and tumor length, were all reduced [79]. Nevertheless, curcumin showed a limited impact in pancreatic cancer patients, despite improving tumour markers, expression of pSTAT3, COX-2, and NF-ƙB, and serum cytokines, which may be linked to the advanced stage of the disease [80]. However, curcumin has promising therapeutic promise since it modulates cancer progression through several pathways, including p53, Bcl-2, PTEN, Notch, and Akt and by altering the production of numerous microRNAs [81]. The development of lung cancer cells and colonies was seen to be inhibited by L48H37, a new curcumin analogue [82].
3.1.9. Curcumin and Male Reproductive System
One of curcumin's primary targets is the male reproductive system [3]. A deleterious impact on the testis is caused by the fact that the anti-parasitic medication metronidazole inhibits testosterone production [83]. Curcumin mitigated some of the side effects of metronidazole, including Leydig cell hyperplasia in the testis and a reduction in the volume, length, width, and height of the germinal epithelium of the tubules. The antioxidant properties of curcumin, as well as its potential to increase blood testosterone levels, have been studied [83]. The curative benefits of curcumin on rats' testicular injury caused by di-nbutylphthalate (DBP) were part of an additional investigation [84, 85]. Polymerizers in cellulose plastics, dye solvents, and a host of other products make heavy use of benzodic acid dibutyl ester, which goes by several different names, including butylphthalate and di-n-butylphthalate (DBP) [85]. A decrease in spermatozoa defects and enhanced sperm motility may result from curcumin's ability to inhibit peroxidative modification in the testicular membrane and sperm. One possible mechanism by which curcumin reduces oxidative damage induced by DBP or its metabolites is by enhancing the inhibition of antioxidant enzymes, G6PD and g-GT, or by stimulating their production [85]. Histopathological examination of testes treated with DBP revealed significant degeneration of seminiferous tubules and extensive necrosis of testicular epidermal cells [85]. When compared to the control group, curcumin maintained the structural and functional activity of seminiferous tubules to some extent, indicating that it had a protective influence on the testes. Curcumin therapy decreased aflatoxin-induced sperm reduction, immobility, and viability, and increased the morphological properties of the sperm [86].
3.1.10. Curcumin and the Female Reproductive System
A woman's reproductive system includes her uterus, vagina, external genitalia, paired ovaries and oviducts, and mammary glands. Ovulation, sperm fertilization, and pregnancy are the three main purposes for which all of these organs have developed physically and physiologically. A study assessed the toxicity of the organophosphate insecticide chlorpyrifos (CPF) in mice after oral exposure [87]. Results indicated that mice infertility was caused by increased lipid peroxidation and decreased FSH hormone levels after CPF administration, which led to inappropriate ovulation. However, fertility was restored in mice after curcumin treatment because lactoperoxidase levels were restored to greater amounts. Curcumin therapy restored normal architecture to the graffian follicle and germinal epithelium in mice, while CPF degenerates both structures, leading to histological alterations [87]. Another study showed the growth-inhibitory impact of curcumin on ovarian cancer in humans. They found that different doses of curcumin significantly suppressed the formation of malignant tumours in the ovary [88]. Some cancer cells exhibited telltale signs of cell death, such as altered shape. One of its molecular mechanisms may have been the stimulation of apoptosis through the down-regulation of Bcl-2 and p53 expression [88].
3.2. Antiviral activity
Curcumin has extensive antimicrobial action, including antibacterial, antiviral, antifungal, and antimalarial activities, as shown in several investigations. Given its prolonged antimicrobial efficacy and safety profile, even at elevated doses (12 g/day) as demonstrated in human clinical trials, curcumin served as a structural template for the development of novel antimicrobial agents with enhanced and modified activities through the synthesis of various curcumin derivatives [89]. Curcumin contains graphene oxide, which has a synergistic antiviral effect when used for the treatment of resveratrol infection. Furthermore, resveratrol infects the lower respiratory tracts of neonates, resulting in severe pulmonary illness. Curcumin has a dose-dependent antiviral effect [90]. Curcumin inhibits the inosine-monophosphate dehydro- genase (IMPDH) enzyme by either a non-competitive or competitive mechanism. Curcumin participates in viral entry and various phases of the life cycle, except viral RNA replication. Inhibition of IMPDH is necessary for curcumin to exhibit potential antiviral, anti-proliferative, and antiparasitic effects [91].
3.3. Depression and Anxiety
Depression shows up in two forms: anxiety and depression. Numerous clinical research have investigated the impact of orally administered curcumin on mental illnesses. Curcumin was administered orally at dosages between 500 and 1000 mg consistently in these studies, either alone, with BioPerine, or in combination with conventional antipsychotics such as escitalopram, venlafaxine, or fluoxetine [92]. The only experiment excluded was one where curcumin therapy alleviated anxiety but not despair, which was may be attributable to the abbreviated administration period (30 days compared to 5–8 weeks in other research) [93]. In recent decades, other explanations have arisen as the monoamine depletion hypothesis has predominated in the pathophysiology of depression. One individual posits that inflammation has a significant role in the pathophysiology of depression. The parallels between “sickness behavior” and depressive symptoms, including anorexia, reduced locomotor activity, and cognitive impairments, have led to this hypothesis. Some studies indicate a positive correlation between these depressive symptoms and C-reactive protein levels (CRP) [94].
Curcumin mitigated coughing, rhinorrhea, and cold symptoms by decreasing nasal airflow resistance. It diminishes intercellular adhesion molecules and decreases IL-4, IL-8, and tumor necrosis factor alpha (TNF-α) while increasing IL-10 levels. Curcumin alleviated allergic airway irritations via the nasal pathway in mice with allergic asthma while preserving structural integrity [95]. The antioxidant properties of curcumin are associated with its enhanced anti-diabetic effects [96].
4. NEGATIVE EFFECT OF CURCUMIN
Curcumin's limited bioavailability, caused by inadequate absorption, rapid metabolism, and quick elimination, is a significant disadvantage despite its anti-inflammatory and antioxidant characteristics (Fig. 3) (Sohn et al., 2021). Various parameters have been examined to improve the bioavailability of curcumin by specifically targeting distinct routes. The primary purpose was to enhance the bioavailability of curcumin by inhibiting the metabolic route responsible for its production [35]. Piperine, for instance, is a well-established compound that enhances the bioavailability of substances, such as curcumin, by up to 2000%. To address the problem of limited bioavailability, bioavailability-enhancing compounds like piperine are commonly employed, forming curcumin complex [32].
Negative effects of curcumin.
While several reports have shown the beneficial effects of curcumin and its therapeutic potential, many more have demonstrated the negative side effects. Dietary turmeric oleoresin, which is comparable to 79–85% commercial Curcumin, showed unsafe symptoms in rats and mice. In
male rats, the consumption of turmeric oleoresin increased the occurrence of inflammation of the forestomach, colon and cecum, as well as hyperplasia and ulcers; in female rats, it increased the incidence of cecum inflammation. In addition, female rats developed thyroid gland follicular hyperplasia after consuming a turmeric oleoresin-containing diet [97]. It was previously demon- strated that curcumin (2.5 and 5 μg/ml) caused DNA damage to the mitochondrial and nuclear genomes in an in vitro experiment using HepG2 human hepatocellular carcinoma cells [98]. Research has shown that topoisomerase II alteration may be the mechanism by which curcumin mediates DNA damage [99]. Curcumin has been shown to have a pro-oxidant effect, according to studies. This might be because it generates reactive oxygen species (ROS) by permanently altering thioredoxin reductase at higher doses, which is a key factor in carcinogenesis [75]. In addition, research has demonstrated that two unsaturated ketones in curcumin's chemical structure bond covalently with exposed thiol groups of cysteine residues in proteins via the Michael addition, which might explain some of its harmful effects. Curcumin has a few documented modest adverse effects in human studies, such as diarrhoea [100]. Curcumin also led to iron chelation, which in turn produced anaemia in mice that had iron-deficient diets [101]. Additionally, a mouse in in vivo research found that curcumin promoted lung cancer [102]. Additionally, curcumin has been found to have angiogenesis-promoting properties. Accordingly, at lower curcumin doses (0.001 and 0.01 mM, 24 h), a rise in VEFG expression was noticed [103]. In a similar vein, curcumin has been found to hasten healing and shield stomach ulcers from further damage by reducing MMP-9 activity and improving MMP-2 activity [103]. Due to the contradictory nature of the beneficial and harmful characteristics of curcumin, more research must be conducted on its safety, even if the data presented in this study strongly suggests that it may have therapeutic potential against cancer.
The effects of oral administration of curcumin at concentrations of 1,500, 3,000, and 10,000 ppm on two generations of Wistar rats [104]. The rats in the F0 and F1 generations received doses of 847 and 959 mg/kg body weight per day, respectively, while the female rats received doses of 1,043 and 1,076 mg/kg body weight per day. The results showed no reproductive toxicity. However, at the 10,000 ppm dose, there was a slight decrease in chicks' body weight gain. Intravenous administration of pegylated curcumin once daily for ten days in male nude mice did not affect. There was a loss in weight of seminal vesicles, a drop in testicular testo- sterone levels, and disruption of spermatogenesis [104].
The properties and oral absorption of curcumin vary in nanoformulations, which curcumin can use in nano- formulations for a variety of applications nowadays. No abnormalities or deaths were seen in rats after 14 days of oral (gavage) administration of nanoparticles. Neither necroscopy nor pathological examinations of the vital organs revealed any signs of poisoning. A single oral dosage of 2,000 mg/kg of curcumin nanoparticles was therefore shown to be safe [105].
4.1. Curcumin and Cytotoxic Effects
The cytotoxic effects of curcumin were greater on cancer cells compared to normal cells. Curcumin (5 to 80 nmol/mL concentration) showed a greater uptake by cancer cells compared to normal cells, suggesting that the cytotoxic effects of curcumin on cancer cells are greater than on normal cells, according to a study that compared cancer cells (EL4 and MCF7) and normal cells (spleen lymphocytes and NIH3T3) [106]. Curcumin inhibited cell proliferation at doses of more than 10 μM and lowered the number of viable cells at concentrations ranging from 1 to 50 μM, according to studies conducted on human epithelial pigment cells [107]. At a dosage of 5 μM, Yang et al. (2006) reported that curcumin reduced cell growth and exhibited toxic effects on normal cells when applied to endothelial cells and vascular smooth muscle cells (VSMCs) isolated from the aorta. Death of VSMCs occurred at 10-μM curcumin. Additionally endothelial cell proliferation can be inhibited at 2.5 μM curcumin, but VSMC proliferation was unaffected at concentrations below 1 μM, while endothelial cell proliferation did drop marginally [108].
Exposure to curcumin at a dose of 20 μM decreased maturation, fertilization, growth, and fetal weights in oocytes from ICR albino mice [109]. The number of blastocyst cells was also much smaller and the cleavage of oocytes to two cells was reduced compared to the control groups. Additionally, the apoptosis of blastocysts was shown to be higher at a dose of 20-μM curcumin. Research conducted on fertilized oocytes in live organisms found that the implantation rate of blastocysts was reduced in female ICR mice when given a normal diet and water with 10 to 40 μM of curcumin. Furthermore, compared to those that did not receive curcumin, blastocyst development after implantation and lifespan were reduced [109]. Cell viability and proliferation were decreased, and the death rate was twice as high at a 100 μg/ml dosage of curcumin [110]. Accordingly, curcumin can decrease cell viability and prevent normal cells from proliferating [110].
4.2. Human Randomized, Placebo‐controlled Clinical Trials: Safety and Toxicity Evaluation
Patients with ulcerative colitis who took 2 grams of curcumin orally once a day for six months did not have any serious side effects; however, they did experience flatulence [111]. In a further investigation involving the same subjects, 180 mg of curcumin was given orally once daily for eight weeks. One participant noticed hyper- tension and tachycardia, while another participant noted redness of the tongue; nevertheless, no serious toxicity was recorded in this study [112]. One patient complained of itching and two patients had constipation when using 1.5 g/day of curcumin for three months; however, no major side effects were noted [113]. Some 240 individuals with type 2 diabetes mellitus experienced nausea and constipation after taking 1,500 mg of curcumin orally daily for six months, but no serious adverse effects were reported [113]. Some individuals who experienced pruritus due to sulphur mustard and took 1 g of curcumin daily for four weeks experienced gastrointestinal side effects.
Two metabolic syndrome patients had nausea and diarrhoea after taking 1,890 mg of curcumin extract orally for 12 weeks [114]. One patient out of fifty-six with severe depressive illness had stomachache and flatulence within eight weeks of taking 1,000 mg of curcumin daily [115].
In a study conducted, patients with sulphur mustard-induced pulmonary problems who took 1.5 g of curcumin daily for four weeks experienced some side effects like constipation, stomachache, and headache. However, no serious toxicity or serious adverse effects were reported. Despite a small amount of gastrointestinal distress, no serious side effects were noted after 8 weeks of oral curcumin administration to patients with solid tumors [116, 117]. For eight weeks, participants in a double-blind study with coronary artery disease were given either 500 mg of curcumin or a placebo in the form of capsules. Just two participants in this research reported diarrhoea and other gastrointestinal issues. Furthermore, when com- pared with the placebo group, there was no significant change in urea, creatinine, and high-density lipoprotein cholesterol in this trial [118]. Turmeric and curcumin, when taken as a standardized powder or extract, were found to be non-toxic to humans in these studies. Additionally, bioavailable formulations like nano- formulations were found to be safe, although there is a need for more research on these formulations and a thorough investigation of their toxicity.
4.3. Challenges in the Application of Curcumin
Curcumin has significant promise in the treatment of human diseases; nevertheless, challenges in its thera- peutic use are equally notable.
Curcumin demonstrates very low water solubility, little absorption in the small intestine, and fast excretion by the liver. Animal studies indicated that upon intake by rats, the majority of curcumin was eliminated in feces in the prototype. Human investigations indicate that the maximum plasma concentration of curcumin reaches only 11.1 nmol/L 1–2 hours after the ingestion of 3.6 g of curcumin. Consequently, the limited bioavailability of curcumin significantly restricts its therapeutic uses, prompting attempts to enhance its bioavailability [95].
Piperine enhances curcumin absorption, inhibits its glucuronidation, and increases its bioavailability by almost 20-fold [35]. Lycopene enhances the antioxidant properties of curcumin and significantly improves its antioxidative effects in mice subjected to acute ethanol oxidative damage [119]. Glycyrrhetinic acid, in conjunction with curcumin, may more effectively suppress the growth of liver cancer cells and cause apoptosis. Metal ions, including Zn2+, Cu2+, Mg2+, and Se2+, together with serum albumin, may form complexes with curcumin to enhance its aqueous solubility and bioavailability. These results illuminate the therapeutic use of curcumin [35].
Liposomes are novel nanopharmaceuticals consisting of a lipid bilayer (phospholipid bilayer and cholesterol) encasing an aqueous interior, capable of encapsulating both lipophilic and hydrophilic substances to enhance solubility and permeability, hence increasing bio- availability. Research indicates that curcumin liposomes formulated with phospholipids, cholesterol, and Tween-80 may enhance stability across diverse pH levels and metal ions, while also improving cellular absorption and antioxidant capabilities [120]. Lecithin-based curcumin liposomes may extend the plasma half-life. Curcumin liposomes, using a blend of phospholipids, sodium hydroxide, and ethanol, may enhance the stability of biological storage. Curcumin liposomes formulated with soy lecithin, nonionic surfactants, and cholesterol may decelerate the continuous release of curcumin [121]. Borneol-modified curcumin cationic liposomes may markedly enhance curcumin levels in vivo and in cerebral tissues, while also prolonging its removal after nasal delivery [122]. Curcumin can be integrated with liposomes, polysaccharides, or biocompatible proteins exhibiting low immunogenicity. This biopolymerization nanoparticle can be encapsulated within granules through hydrophobic and hydrogen bonding, facilitating cell endocytosis or enhancing direct absorption by small intestinal epithelial cells. This process inhibits the oxidative decomposition of curcumin in the intestine, thereby improving its solubility and bioavailability. For instance, chitosan-curcumin exhibits superior antioxidant and metal chelating properties [123].
CONCLUSION
Curcumin has numerous positive effects on health. It exhibits antioxidant and anti-inflammatory properties, which contribute to its ability to manage conditions, including inflammatory and progressive eye conditions, cancer, metabolic syndrome, skin diseases, neuro- degenerative diseases, liver diseases, and pain. Curcumin supplementation improves endothelial function, reduces oxidative stress, and inhibits the activation of inflammatory signalling pathways. Overall, curcumin has a wide range of health benefits and can be an invaluable addition to a healthy lifestyle. Although curcumin has numerous medicinal benefits, such as anti-inflammatory and antioxidant properties, it also has several negative effects. These include limited bioavailability, DNA damage, pro-oxidant effects, adverse effects such as diarrhoea, iron chelation leading to anaemia, promotion of lung cancer and angiogenesis, and cytotoxic effects on both cancer cells and normal cells. Human randomized, placebo-controlled clinical trials reported minor side effects such as flatulence, hypertension, tachycardia, redness of the tongue, itching, constipation, nausea, and gastrointestinal issues in subjects. Overall, curcumin appears to be a safe and promising compound with potential health benefits. However, current understanding warrants more research exploring its mechanisms of action, optimal dosages, and potential interactions with other medications or health conditions.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| 6-OHDA | = 6-hydroxydopamine |
| ACh | = Acetylcholine |
| AST | = Aspartate transaminase |
| BDMC | = Bisdemethoxycurcumin |
| CPF | = Chlorpyrifos |
| DMC | = Desmethoxycurcumin |
| DA | = Dopamine |
| FBG | = Fasting blood glucose |
| FDA | = Food and Drug Administration |
| FFAs | = Free fatty acids |
| GRAS | = Generally Recognized as Safe |
| HSP70 | = Heat shock protein 70 |
| HbA1c | = Hemoglobin A1c |
| HCC | = Hepatocellular carcinoma |
| LPL | = Lipoprotein lipase |
| MDA | = Malondialdehyde |
| NGF | = Nerve growth factor |
| Nrf2 | = NF-E2-related factor 2 |
| NAFLD | = Non-alcoholic fatty liver disease |
| NASH | = Non-alcoholic steatohepatitis |
| OA | = Osteoarthritis |
| P-gp | = Permeability glycoprotein |
| ROS | = Reactive oxygen species |
| RA | = Rheumatoid arthritis |
| SLNS | = Solid lipid nanoparticles |
| SOD | = Superoxide dismutase |
| TG | = Triglyceride |
| T2DM | = Type II Diabetes Mellitus |


