All published articles of this journal are available on ScienceDirect.
Bioactive Polyphenolic and Terpenoid Compounds from Stem Bark and Flowers of Terminalia brownii
Abstract
Introduction
Terminalia brownii Fresen (Combretaceae) extensively used in Eastern, Southern, and Western Africa herbal remedies in treatment of variety of diseases including liver cirrhosis. Bioassay – guided fractionation was used to isolate the compounds responsible for these actions.
Aim of the Study
The study sought to extract, characterize and determine the antimicrobial activities of the components of the commonly used stem bark and hitherto uninvestigated regeneratable flowers of T.brownii.
Materials and Methods
Column chromatography was used to fractionate and isolate the compounds followed by thin layer chromatography. The isolates were structurally elucidated using FTIR, 1H NMR, 13C NMR and HRESI-MS spectral data. The isolated compounds' antimicrobial activities were evaluated against Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli.
Results
Bioassay – guided fractionation of methanol and 50% dichloromethane: methanol extracts of Terminalia brownii stem bark and flowers yielded seven known metabolites; termiglaucescin (1), arjunglucoside-I (2), sericoside (3), 23-galloyl arjungenin (4), 28-O-β-D-glucopyranosyl-2,3,6-trihydroxy-23-galloylolean-12-dien-28-oate (5), 3,3',4',5-tetrahydroxy-7-methoxyflavone (9), 3,3',4',5,7-pentahydroxyflavone (10) and three new metabolites: 1,4,7-tri-O-galloyl hept-6-deoxyheptose (6), 1,2,4-tri-O-galloyl-8,9-dideoxynonose (7), Rhamnetin-3-O-(2,3,6-trigalloyl)-β-D-glucopyranoside (8). Among these molecules, compound (6) was extremely potent toward E. coli (16.5±0.7 mm) while (1) showed substantial inhibitory effects on Candida albicans (16.0±5.7 mm). Compounds 6, 7, 8, 9, and 10 were extracted and identified for the first time from 50% dichloromethane/methanol flower extract. The extract yielded three novel bioactive compounds (6, 7 and 8) that shown substantial activity on P.aeruginosa, E. coli, C. albicans, S. aureus.
Conclusion
For the first time, the results of this investigation demonstrate that flower extract possess strong antibacterial and antifungal qualities, akin to those of stem bark extract. As a result, more environmentally friendly flower extracts should be considered for treatment of bacterial and fungal infection.
1. INTRODUCTION
The management of prevalent illnesses such as flu, fatal acute respiratory syndrome and diarrhea remains jeopardized due to the advent of multidrug resistance pathogens [1]. It’s estimated that infectious diseases account for almost half of all deaths in tropical countries [2]. Plants constitute a storehouse of naturally occurring metabolites with medicinal traits and they have been extensively utilized in treating an array of maladies around the globe since time immemorial [3, 4] hence they are regarded as goldmine for development of potent pharmaceuticals [5]. Plant-based drugs are believed to be secure and cost – effective when juxtaposed with artificial drugs/conventional medicine and thus, their demand is rapidly increasing. Even though multiple studies have certified the use of plants for medical purposes through exploring secondary metabolites found in them, barely 15% of available medicinal plants have undergone scientific investigation to ascertain their phytochemical makeup and potential for medicinal purposes [6-8].
The genus Terminalia (Combretaceae) has a diverse range of plants prevalent in tropical and subtropical climates across the globe, encompassing Africa, Asia and Australia [9]. The trees are primarily employed in ancient systems of medicine across different cultures providing health benefits and treatment of various ailments such as geriatric, wound healing, flu, reducing oxidative stress and inflammation in the body, improve digestion, improve memory, relieve constipation and promote bowel movements, regulate blood pressure, managing blood sugar levels, management of dental caries and gum diseases [10, 11]. The prominent secondary metabolites that have been confirmed from this genus are triterpenoids, tannins, glycosidic derivatives and flavonoids. A few are reported to embody diverse biological traits notably antiretroviral, antibacterial, antifungal, antimalarial and antioxidant effects [12]. T.brownii has been used to manage a wide range of ailments notably flu, geriatric and back pain [13]. Concoctions of roots are utilized in management of back pain while chewing of stem bark relieves cough and arthritis [14]. Notwithstanding the reported ethnopharma- cological usage of various Terminalia species, very few research have been carried out to comprehensively investigate the medicinal potential of different parts of T.brownii. This study aimed at isolation and structural elucidation of isolates from T.brownii stem bark and flowers. The obtained isolates were also tested for antibacterial activity against bacterial pathogens.
2. METHODOLOGY
2.1. Plant Materials, Extraction and Isolation
Stem bark and flowers of T. brownii were collected in April, 2021 in Mwingi; Kitui County, Kenya (1°02'18.7”S 37°55'01.9”E) and identified at the Herbarium of South Eastern Kenya University (voucher number SKU.JULY/2023/01). The plant name was checked with the plant list database (http:/www.theplantlist.org) to confirm plant taxonomy. The air – dried plant materials were powdered and sequentially extracted using n-hexane, dichloromethane, 50% dichloromethane/methanol and methanol through maceration [15].
50% dichloromethane/methanol and methanol extracts of T. brownii’s stem bark and flowers which exhibited strong antimicrobial activity [16], were separately subjected to column chromatography on a silica gel and eluted with gradient of ethyl acetate/n-hexane (40:60, 50:50, 60:40, 70:30, 80:20, 90:10, 100.0, v/v) and methanol/ethyl acetate (5:95, 10:90, 15:85, 20:80, v/v). The fractions were purified by preparative thin layer chromatography and pure isolates characterized using 1H NMR, 13C NMR, IR, and HRESI-MS.
3. RESULTS AND DISCUSSION
3.1. Isolated Compounds
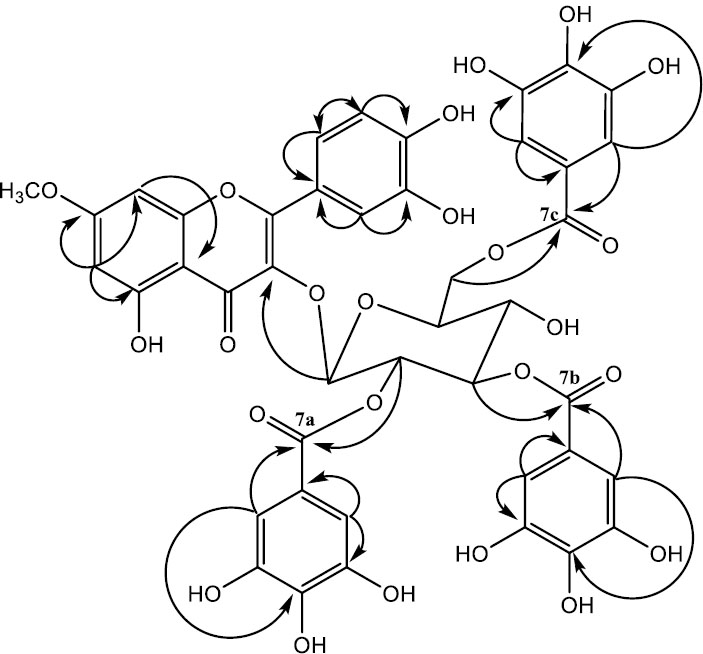
Bioassay-guided study of 50% dichloromethane/methanol and methanol fractions led to separation and detection of seven known metabolites and three new compounds. The isolates were assessed using 1H NMR, 13NMR,IR, HRESI-MS and results compared with previously reported data for the seven known compounds, termiglaucescin, 69 mg (1) [17], arjunglucoside – I, 130 mg (2) [18], sericoside, 23 mg (3) [19], 23-galloyl arjungenin, 95 mg (4) [17], 28-O-β-D-glucopyranosyl-2,3,6-trihydroxy-23-galloylolean-12-dien-28-oate, 48 mg (5) [20], 3,3',4',5 tetrahydroxy-7-methoxy flavone, 61 mg (9) [21] and 3,3’,4’,5,7-penta hydroxy flavone, 98 mg (10) [22]. The three newly isolated compounds were identified as 1,4, 7-tri-O-galloyl-6-deoxyheptose, 11mg (6) [23] 1,2,4-tri-O-galloyl-8,9 dideoxynonose, 10 mg (7) [24] and rhamnetin-3-O-2,3,6-tri-galloyl-β-D-glucopyranoside, 40 mg (8) [25]. Fig. (1) illustrates the molecular structures of the isolates.
Compounds 1 – 3 and 4 -5 were isolated from methanol and 50% dichloromethane/methanol extract of T.brownii stem bark respectively while compound 6 – 10 were obtained from 50% dichloromethane:methanol extract of T.brownii flower. Compound 1, 4,5,6,7 and 8 were eluted by 15% methanol/ethyl acetate while compound 2, 3, 9 and 10 were eluted by 20% methanol/ethyl acetate solvent system.
Compound 6 identified as 1,4, 7-tri-O-galloyl-6-deoxyheptose was isolated as light brown amorphous substance (11 mg) with melting point range of 176 – 178 °C and molecular formula C28H26O18 as determined by HRESIMS (m/z 650.1106). The infrared Absorption at 3295, 1708 and 1611 cm-1 revealed existence of hydroxyl, ester carbonyl and C=C functional group respectively in the molecule.
Compound 6 was generated based on spectral data evaluation [23, 26]. C – 6 substitution evidenced by lack of hydroxyl proton at C-6, 1H NMR spectrum (Fig. 2) which is typically diagnostic of hydroxyl group on C – 6 in heptose [27, 28]. The 1H NMR signal at C – 1, C – 4 and C – 7 of heptose moiety were significantly downfield compared to those of 6-deoxyheptose [23]. 13C NMR spectrum (Table 1) exhibited seven aliphatic carbon indicating presence of 6-deoxyheptose and 15 sp2 carbon signals corresponding to three galloyl groups. COSY and HMBC correlations (Fig. 3) revealed the atomic connectivity within the 6-deoxyheptose framework and confirmed the substitution pattern, supporting the identification of Compound 6.
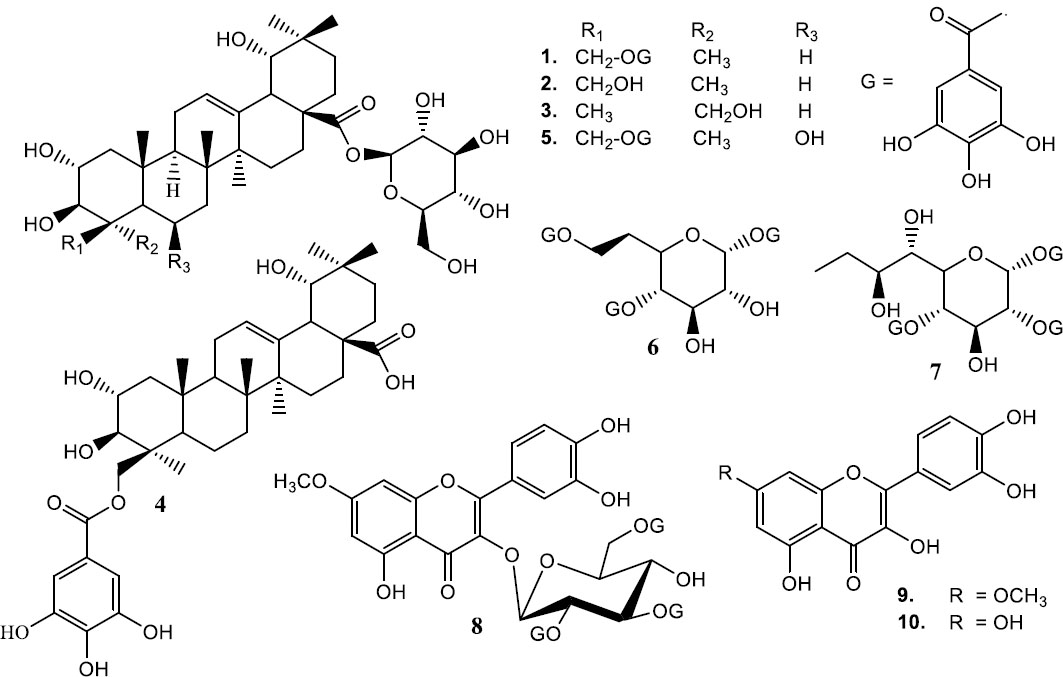
Molecular structures of compounds 1-10.
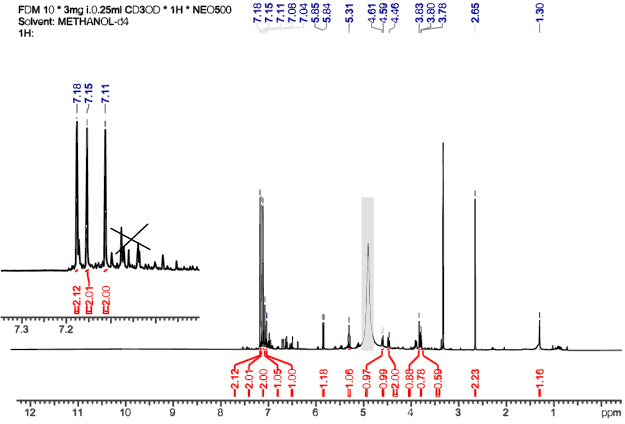
1 HNMR spectrum of structure 6 (MeOH d4, 500 MHz).
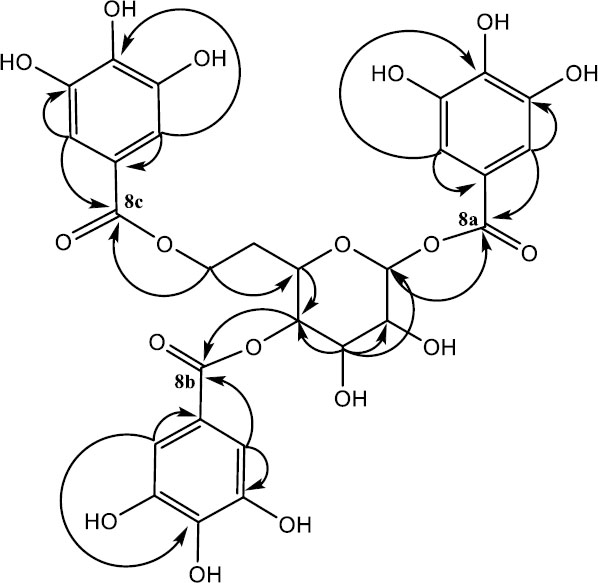
Key H/C HMBC correlations of compound 6.
Compound 7 for 1,2,4-tri-O-galloyl-8,9 dideoxynonose was retrieved as brown powder (10 mg) with melting point range of 186 – 188 oC and molecular formula C30H30O18 as determined by HRESIMS analysis (m/z 678.1536). The identification of compound 7 was based on spectral data analysis [24, 29]. The 1H NMR spectrum (Fig. 4) three aromatic proton signifying existence of three galloyl units. In the sugar regions, three protons were observed significantly downfield compared to those of nonose moiety implying the attachment of galloyl entities to their respective carbons [30]. 13C NMR spectrum (Table 1) revealed eight aliphatic carbons associated with 8,9 dideoxynonose moiety and 15 sp2 carbon representing the three galloyl groups. HMBC correlations (Fig. 5) showed the inter – atomic interaction of 8,9 dideoxynonose and galloyl skeletons and the substitution pattern of the 8,9 dideoxynonose system, thus confirming compound 7.
1 HNMR spectrum of structure 7 (MeOH d4, 500 MHz).
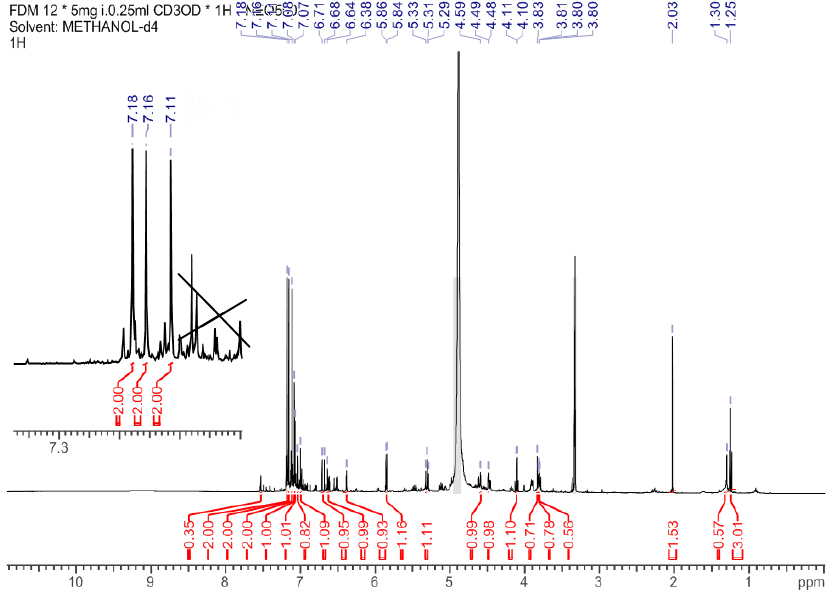
Compound 8 for rhamnetin-3-O-(2,3,6-tri-galloyl)-β-D-glucopyranoside was acquired as pale green powder (40 mg) with melting point range of 156 – 157 °C and molecular formula C43H34O24 as confirmed by HRESIMS analysis (m/z 934.1524). Infrared absorption at 3201, 1705 and 1607 cm-1 confirmed the existence of hydroxyl, carbonyl and C-C stretching at aromatic system respectively. The compound was established based on spectroscopic data analysis [25, 31]. The 1H NMR signal (Fig. 6) at C – 2 and C – 3 of the glucosyl moiety were significantly downfield compared to those of rhamnetin-3-O-6-galloyl)-β-D-glucopyranoside [31, 32]. The 13C NMR data (Table 2) indicated three galloyl groups. HMBC correlations interactions (Fig. 7) revealed inter – atomic interactions in rhamnetin-3-O-6-galloyl)-β-D-glucopyranoside framework and substitution on glucosyl moiety, confirming the structure of compound 8.
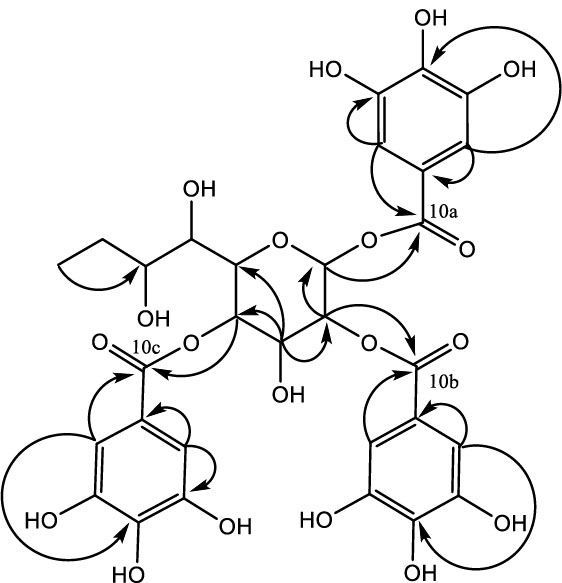
Key H/C HMBC correlations of compound 7.
| Structure 6 | Structure 7 | ||||||
|---|---|---|---|---|---|---|---|
| S/No. | 13C (ppm) | 1H NMR δ (ppm) | HMBC Correlations | S/No. | 13C | 1H δ(ppm) | HMBC Correlations |
| 1 | 94.5 | 5.9 (d, 1H, 8.2) | 3, 8a | 1 | 94.5 | 5.9 (d, 1H, 8.2) | 10a |
| 2 | 71.2 | 3.8 (s, 1H) | - | 2 | 71.2 | 3.8 (d, 1H, 1.2) | 1, 3, 5, 10b |
| 3 | 75.0 | 3.8 (d, 1H, 1.2) | 1, 2, 4, 5 | 3 | 75.0 | 3.8 (d, 1H, 3.1) | 4, 6 |
| 4 | 68.3 | 3.8 (m, 1H) | 2, 3, 8b | 4 | 68.3 | 3.8 (m, 1H) | 1, 3, 5, 6, 10c |
| 5 | 77.5 | 5.3 (t, 2H, 9.3) | 1, 2, 3, 4, 8b | 5 | 77.5 | 5.3 (d, 1H, 9.3) | 2, 4, 10c |
| 6 | 39.0 | 2.7 (s, 2H) | - | 6 | 62.8 | 4.6 (d, 1H, 1.8) | - |
| 7 | 62.8 | 4.5 (dd, 2H, 12.1; 4.8) | 3, 4, 5, 7c, 8c | 7 | 60.2 | 4.5 (d, 1H, 4.7) | - |
| Galloyl unit – 1 | 8 | 19.5 | 2.0 (s, 2H) | - | |||
| 8a | 165.4 | - | - | 9 | 13.1 | 1.3 (t, 3H, 7.2) | 7 |
| 9a | 120.2 | - | - | Galloyl unit – 1 | |||
| 10a | 109.0 | 7.2 (s, 2H) | 8a, 9a, 11a, 12a | 10a | 165.5 | - | - |
| 11a | 145.1 | - | - | 11a | 120.2 | - | - |
| 12a | 138.4 | - | - | 12a | 109.0 | 7.2 (s, 2H) | 10a, 11a, 13a, 14a |
| Galloyl unit – 2 | 13a | 145.0 | - | - | |||
| 8b | 166.7 | - | - | 14a | 138.4 | - | - |
| 9b | 119.1 | - | - | Galloyl unit – 2 | |||
| 10b | 108.9 | 7.1 (s, 2H) | 8b, 9b, 11b, 12b | 10b | 169.0 | - | - |
| 11b | 145.0 | - | - | 11b | 120.6 | - | - |
| 12b | 139.1 | - | - | 12b | 109.2 | 7.2 (s, 2H) | 10b, 11b, 13b, 14b |
| Galloyl unit – 3 | 13b | 145.1 | - | - | |||
| 8c | 166.8 | - | - | 14b | 138.2 | - | - |
| 9c | 119.9 | - | - | Galloyl unit – 3 | |||
| 10c | 109.2 | 7.2 (s, 2H) | 8c, 9c, 11c, 12c | 10c | 166.8 | - | - |
| 11c | 145.1 | - | - | 11c | 119.1 | - | - |
| 12c | 138.5 | - | - | 12c | 108.9 | 7.1 (s, 2H) | 10c, 11c, 13c, 14c |
| - | - | - | - | 13c | 145.0 | - | - |
| - | - | - | - | 14c | 139.1 | - | - |
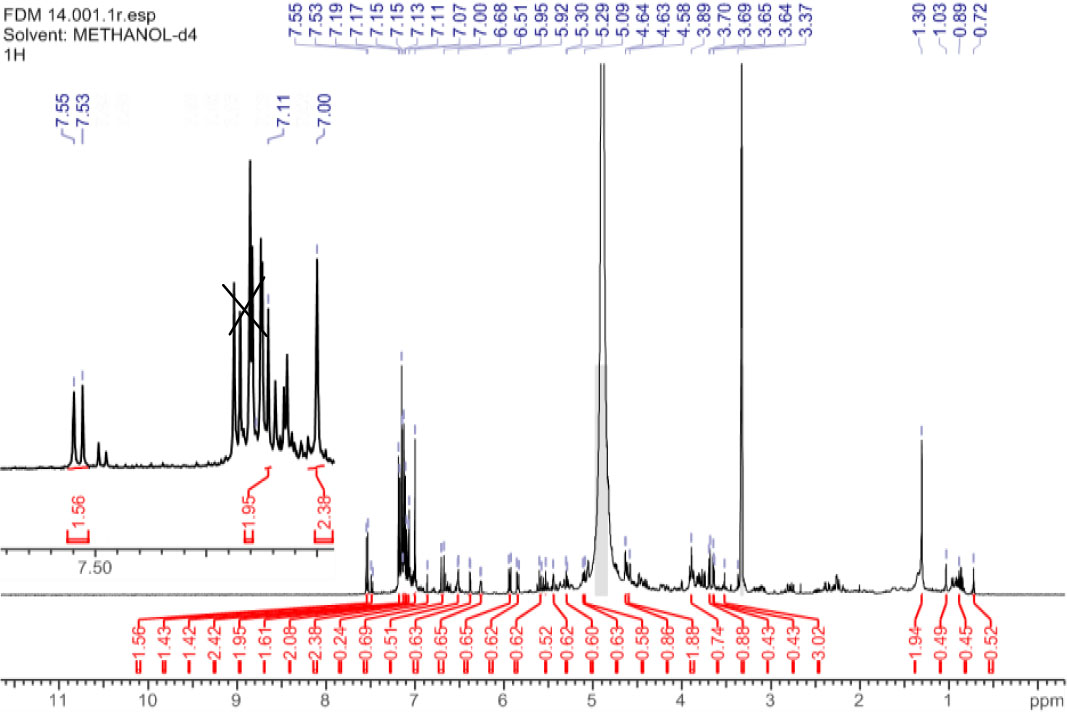
1 HNMR spectrum of structure 8 (MeOH d4, 400 MHz).
Key H/C HMBC correlations of compound 8.
| S.No. | 13C δ(ppm) | 1H δ(ppm) | HMBC Correlations | ||
|---|---|---|---|---|---|
| 2 | 148.2 | - | - | ||
| 3 | 136.3 | - | - | ||
| 4 | 173.5 | - | - | ||
| 5 | 164.7 | - | - | ||
| 6 | 97.1 | 6.3 (d, 1H, 1.2) | - | ||
| 7 | 168.9 | - | - | ||
| 8 | 94.5 | 6.4 (d, 1H, 1.6) | - | ||
| 9 | 164.9 | - | - | ||
| 10 | 108.9 | - | - | ||
| 7 O-CH3 | 51.8 | 3.3 (br s, 1H) | 168.9 | ||
| 1’ | 121.4 | - | - | ||
| 2’ | 109.3 | 7.2 (d, 2H, J = 5.4) | - | ||
| 3’ | 145.1 | - | - | ||
| 4’ | 148.3 | - | - | ||
| 5’ | 109.0 | 5.8 (d, 1H, 8.3) | - | ||
| 6’ | 109.3 | 7.1 (dd, 1H, 8.3, 2.2) | - | ||
| Sugar unit | |||||
| 1” | 106.9 | 6.7 (br d, 1H, 0.9) | 136.3 | ||
| 2” | 72.7 | 4.2 (br d, 1H, J = 0.9) | 164.9 | ||
| 3” | 76.9 | 4.6 (d, 1H, J = 1.3) | 166.6 | ||
| 4” | 70.3 | 5.4 (m, 1H) | - | ||
| 5” | 75.0 | 5.6 (m, 1H) | - | ||
| 6” | 62.5 | 4.8 (br s, 2H)) | 166.5 | ||
| Galloyl unit – 1 | |||||
| 7a | 164.9 | - | - | ||
| 8a | 120.7 | - | - | ||
| 9a | 110.2 | 7.6 (d, 2H, 8.0) | 120.7, 139.9, 145.0, 164.9 | ||
| 10a | 145.0 | - | - | ||
| 11a | 139.9 | - | - | ||
| Galloyl unit – 2 | |||||
| 7b | 166.6 | - | - | ||
| 8b | 120.0 | - | - | ||
| 9b | 109.1 | 7.1 (s, 2H) | 120.0, 138.8,145.1, 166.6 | ||
| 10b | 145.1 | - | - | ||
| 11b | 138.8 | - | - | ||
| Galloyl unit – 3 | |||||
| 7c | 166.5 | - | - | ||
| 8c | 119.0 | - | - | ||
| 9c | 108.8 | 7.0 (s, 2H) | 119.0, 138.7, 145.5, 166.5 | ||
| 10c | 145.5 | - | - | ||
| 11c | 138.7 | - | - | ||
| Zone of Inhibition in mm (mean ± SD) | ||||
|---|---|---|---|---|
| Isolated Compounds | E. coli | P. aeruginosa | S. aureus | C. albicans |
| 1 | 10.50±0.7 | 10.8±1.1 | 10.5±0.7 | 16.00±2.7 |
| 2 | 10.5±0.7 | 10.0±2.8 | 12.5±0.7 | 15.0±2.2 |
| 3 | 8.5±0.7 | 8.5±0.7 | 10.8±1.1 | 13.0±1.4 |
| 4 | 9.3±0.4 | 9.5±0.7 | 12.3±0.4 | 5.5±1.8 |
| 5 | 12.8±0.4 | 11.8±0.4 | 10.5±0.7 | 14.0±1.4 |
| 6 | 16.5±0.7 | 9.5±0.7 | 10.0±1.4 | 10.5±0.7 |
| 7 | 12.5±0.7 | 13.0±2.7 | 10.0±2.8 | 12.5±2.5 |
| 8 | 10.5±0.7 | 8.0±2.8 | 11.5±0.7 | 11.5±0.7 |
| 9 | 9.5±2.1 | 11.5±0.7 | 10.5±0.7 | 12.8±1.1 |
| 10 | 10.5±0.7 | 9.5±0.7 | 8.8±1.1 | 11.8±0.4 |
| Amoxillin | 15.2±2.4 | 20.0±0.7 | 15.3±2.2 | - |
| Fluconazole | - | - | - | 23.0±5.7 |
| Distilled water | - | - | - | - |
3.2. Antimicrobial Activity
All isolated compounds showed activity against the tested pathogens (Table 3). E. coli, was more susceptible to compound 6 (16.5±0.7). Compound 1 exhibited excellent antifungal activity, with zone of inhibition of 16.0±2.7 mm. All the identified compounds had reduced antimicrobial efficacy compared to the positive control. According to [33], plant extracts containing flavonoids and phenolic exhibit antibacterial activity against a wide spectrum of harmful bacteria due to their chemical structures and biological properties. Flavonoids and phenolics contain hydroxyl group that interact with bacterial cell wall and membranes, creating disruption of cell integrity, inhibition of enzyme function and interference with bacterial DNA and protein synthesis [34]. Additionally, these compounds may act as antioxidants reducing oxidative stress in cells inhibiting bacterial growth hence making them effective against various bacterial strains [35, 36]. Terpenes and their derivatives exhibit potent antimicrobial activity against drug – resistant pathogens bacteria and fungi owing to their ability to erode microbial cell membranes and obstruct integral cellular process [13, 37]. These results align with previous studies that documented the antimicrobial properties of termiglaucescin (1), arjunglucoside – I (2), Sericoside (3), 23-galloyl arjungenin (4) [17, 38]. This corroboration reinforces the potential of these compounds as viable option for developing new antimicrobial agent particularly in the face of rising drug resistance.
The study proposes the isolation and characterization of n-hexane, chloroform and ethylacetate extract of T.brownii flowers and carry out toxicity and antioxidant tests on T.brownii flowers extracts.
CONCLUSION
This study investigated the antimicrobial activity of stem bark and flowers of T.brownii, a plant traditionally used in ethnomedicine to treat various ailments. Bioassay guided fractionation through silica gel column chromatography, three new compounds were isolated and characterized 1,4, 7-tri-O-galloyl-6-deoxyheptose, 1,2,4-tri-O-galloyl-8,9 dideoxynonose and rhamnetin-3-O-(2,3,6-tri-galloyl)-β-D-glucopyranoside alongside seven existing compounds.
Evaluation of antimicrobial activities of the identified compounds against the test pathogens indicated that compounds 1 – 5 demonstrated relatively high antimicrobial effects, implying that terpenes and their derivatives constitute promising candidate for development of antimicrobial drugs targeting both susceptible and resilient pathogens. The findings underscores the potential of T. brownii as a repository of bioactive compounds with promising antimicrobial properties. The results support the plant’s traditional uses in treating infections and highlight its value in developing new antimicrobial agents. In conclusion this study has demonstrated that flower extracts possess potent antimicrobial properties comparable to those found in stem bark. As a result, more environmentally friendly flower extracts should be considered for treatment of bacterial and fungal infection.
AUTHORS' CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| COSY | = Correlation Spectroscopy |
| HMBC | = Heteronuclear Multiple Bond Correlation |
| HRESMS | = High – Resolution Electrospray Ionization Mass Spectrometry |
| HSQC | = Heteronuclear Single Quantum Coherence Spectroscopy |
| NMR | = Nuclear Magnetic Resonance |
| FTIR | = Fourier Transform Infrared |
AVAILABILITY OF DATA AND MATERIAL
All data generated or analyzed during this study are included in this published article.
ACKNOWLEDGEMENTS
The authors would like to appreciate Technical University of Mombasa (TUM) for providing laboratory space and Mr. Kennedy Agoi for support in bioassay at TUM.


