All published articles of this journal are available on ScienceDirect.
Cytotoxicity Evaluation of Green-synthesized MgO Nanoparticles with the Combination of Curcuma, Cardamom, and Pippali for Potential Cardioprotective Therapy in Diabetes-associated CVDs
Abstract
Introduction
Diabetes mellitus increases the risk of Cardiovascular Diseases (CVDs), highlighting the need for new treatments. Herbal remedies like turmeric (Curcuma longa), with its bioactive compound curcumin, are known for their anticoagulant effects. Cardamom (Elettaria cardamomum) helps lower diastolic blood pressure and inflammatory markers linked to CVDs. Pippali (Piper longum) acts as an anti-inflammatory, analgesic, and bioenhancer. In the current study, acknowledging their properties, these three herbs were combined (Curc+Card+Pip) and conjugated with green-synthesized MgO nanoparticles from curry leaves (Murraya koenigii). The aim was to evaluate the cytotoxicity of the herbal combination with and without MgONPs before employing it as a potential novel therapeutic approach for CVD-related research in diabetes.
Methods
This case-control study included two groups: a) normoglycemic individuals and b) those with Type 2 Diabetes Mellitus (T2DM). Hydroethanolic extracts were prepared and characterized. Cytotoxicity of Curc+Card+Pip and Curc+Card+Pip+MgONps was assessed using the MTT assay over 24 and 48 hours at concentrations of 100-500 µg/ml. Peripheral Blood Mononuclear Cells (PBMCs) from both groups were used as the primary cell lines. Statistical analysis was conducted using SPSS 26.0 and Excel 2021.
Results
The MTT assay on PBMCs from Normoglycemic (NG) and T2DM groups with i) Curc+Card+Pip and ii) Curc+Card+Pip+MgONps showed minimal cytotoxicity at concentrations of 100-500 µg/ml. The CC50 order was NG 24 hours >; T2DM 24 hours >; NG 48 hours >; T2DM 48 hours. A significant difference in cell viability was observed between 24 hours (96.45% ± 0.012) and 48 hours (73.62% ± 0.48) at 100 µg/ml in NG (p >; 0.01), while T2DM showed 93.63% ± 0.012 and 68.41% ± 0.048 at 24 and 48 hours, respectively. For NG, Curc+Card+Pip+MgONps exhibited viabilities of 92.75% ± 0.026 and 62.02% ± 0.046 at 24 and 48 hours, respectively, while T2DM showed 90.82% ± 0.018 and 59.44% ± 0.044.
Discussion
Cytotoxicity increased from 100-500 µg/ml, reducing viability. Cytotoxicity increased at 400-500 µg/ml at 48 hours (p >; 0.001) in both groups and treatments, suggesting that cell tolerance to MgO NPs was similar to that without NPs. The herbal mixture with MgONPs displayed a cytotoxicity pattern similar to that of the mixture without NPs in both groups.
Conclusion
The CC50 for Cur+Card+Pip without and with NPs was 452 and 410 µg/ml, respectively, within 24 hours, whereas in T2DM patients, the same treatments were found to have CC50 values of 300 and 330 µg/ml, respectively, with and without NPs. The values were the same in the case of T2DM (48 hours), whether it was with or without NPs (CC50 = 240 µg/ml), allowing for future exploration of its anti-inflammatory and antioxidant effects on PBMCs, which could suggest its potential as an alternative therapy for managing CVD in diabetic patients.
1. INTRODUCTION
The rising prevalence of T2DM and its associated complications, particularly CVDs, necessitates an urgent need for novel therapies that can mitigate these risks without the harmful side effects of conventional drugs [1]. Herbal medicine has been valued for its therapeutic potential, with various plant extracts demonstrating biological activities that are relevant to modern medicine [2].
Among these, turmeric (Curcuma longa), cardamom (Elettaria cardamomum), and pippali (Piper longum) stand out for their traditional and contemporary use.
Many Preclinical studies have extensively examined the effects of curcumin, the active compound in Curcuma longa, on cardiovascular diseases using rat models or on humans. The studies have demonstrated several cardioprotective properties of curcumin [3, 4].
As in diabetes, chronic inflammation and oxidative stress cause endothelial dysfunction, leading to atherosclerosis and cardiovascular complications. Curcumin's antioxidant properties help neutralize free radicals, reducing these harmful effects [5]. Additionally, curcumin has been shown to improve lipid profiles by lowering LDL cholesterol and triglycerides while increasing HDL cholesterol, thereby reducing the risk of atherosclerosis [6]. Curcumin reduces oxidative stress, inflammation, and endothelial dysfunction in diabetes while enhancing insulin sensitivity and glucose metabolism, thereby aiding in glycemic control and preventing cardiovascular complications [7].
Similarly, Preclinical in vivo studies have explored the cardioprotective effects of cardamom [8, 9].
A meta-analysis revealed that cardamom supplementation reduced inflammatory markers (hs-CRP, IL-6, and TNF-α) and improved blood pressure, highlighting its anti-inflammatory and antioxidant effects [10]. A trial in overweight T2DM patients showed that 3 grams of cardamom daily for 10 weeks improved glycemic indices, triglyceride levels, endothelial function, and reduced inflammation [11].
Studies have even shown that Piperine can improve lipid profiles and enhance insulin sensitivity, both of which are critical factors in cardiovascular health. Additionally, piperine's antioxidant properties may mitigate oxidative stress, a contributor to cardiovascular diseases. Piperine significantly enhances the bioavailability of therapeutic agents by inhibiting metabolic enzymes, enhancing the absorption and effectiveness of co-administered drugs. This is especially useful for improving the efficacy of poorly absorbed compounds, such as curcumin [12-14].
Knowing the medicinal properties of these herbs, it was thought that they could be used as therapeutics for the research related to CVDs in diabetic patients. Investigating the cytotoxicity and therapeutic potential of these extracts is crucial for integrating them into pharmaceutical applications, especially in the management of chronic conditions like Type 2 Diabetes Mellitus (T2DM).
Peripheral Blood Mononuclear cells PBMCs, which include lymphocytes (T cells, B cells, and NK cells) and monocytes, are critical components of the immune system. Studying PBMCs allows researchers to assess the immunomodulatory effects of compounds, including nanoparticles and herbal extracts, which is particularly relevant for understanding systemic toxicity and immune responses [15]. PBMCs can be utilized in assessing the cytotoxic effects of herbs conjugated with nanoparticles, as they serve as an effective primary cell model for MTT assays in evaluating drug responses in T2DM patients due to their relevance to inflammation, insulin resistance, and immune modulation [16].
As per our published literature [17], the herbal hydroethanolic extracts of Curcuma, cardamom, and pippali were made through the Soxhlet apparatus, and combined in this way:
a) Curcuma+Pippali and b) Cardamom+Pippali. Recognizing the importance of nanotechnology and especially green synthesis, the MgO nanoparticles were prepared using curry leaves (Murraya koenigii). Following the characterization of herbal extracts and MgONPs, the conjugation of herbal extracts with MgONPs was achieved by combining them to improve the drug delivery system. The combinations were: c) Curcuma + Pippali + MgONPs and d) Cardamom + Pippali + MgONPs. The two different combinations were subjected to cytotoxicity assay and compared with combinations without NPs. The efficacy of Cardamom + Pippali and Cardamom + Pippali + MgONPs for cell viability was found to be higher compared to Curcuma + Pippali with and without MgONPs [17].
Cytotoxicity testing can be used for quality control purposes. Hence, as a preliminary test before going for further evaluations related to Cardiovascular research, the MTT assay becomes an important tool.
This study builds upon previous research conducted in our laboratory, now aiming to assess the cytotoxicity of a combined formulation of three herbs—Turmeric (Curcuma longa), Cardamom (Elettaria cardamomum), and Pippali (Piper longum)—both with and without MgO nanoparticles (MgO NPs) using the MTT assay. By evaluating cell viability in response to these combinations, we aim to determine their safety profile and potential therapeutic efficacy. Regardless of their therapeutic benefits, only nanoparticles with minimal toxicological risks will be considered for future research and possible human applications. Ultimately, this study could serve as a foundation for future investigations into managing diabetes-related cardiovascular complications, contributing to the development of safer and more effective alternative treatments. Additionally, assessing the cytotoxicity of these herbal combinations with MgO NPs for the first time could help establish a definitive dosage guideline for future research.
2. MATERIALS AND METHODS
2.1. Study Design and Setting
This is a case-control experimental study on T2DM patients and Normoglycemic (NG) individuals.
2.2. Confidentiality of Data
Study subjects were counselled separately about the study, and an informed written consent form was obtained from each patient. Demographic data, like age and gender, were recorded along with a previous medical history using a semi-structured questionnaire. The information concerning the patients was obtained under strict confidentiality.
2.3. Study Period
The study was conducted in Khemdas Ayurveda Hospital, Parul University, Vadodara, from November 2023 to April 2024.
2.4. Patient Selection and Study Sample Size
It was a pilot study, and the sample size was determined using a non-probability, characteristic, and convenience sampling method. Overall, 10 patients with T2DM aged between 20 and 60 years (T2DM group) were recruited from Khemdas Ayurveda Hospital, Vadodara, India. All patients with a T2DM diagnosis followed the ICMR (Indian Council of Medical Research) diabetes guidelines criteria, with a fasting blood glucose level of ≥ 126 mg/dL and an HbA1c level of ≥ 6.5% [18].
In addition, 10 unrelated subjects (NG group) with no previous diagnosis of T2DM, a fasting serum glucose level of ≤ 110 mg/dL, and a similar age group were recruited from the local area in Parul University, Vadodara.
Informed consent was taken from the subjects. The exclusion criteria were pregnancy, alcohol, and not having taken any anti-inflammatory intervention in the last two weeks. The inclusion criteria encompassed non-diabetic individuals and patients with T2DM, aged 20 to 60 years, who resided in Vadodara, Gujarat.
All T2DM patients were on antidiabetic therapy (Metformin), and six of them were on anti-hypertensive drugs (e.g., Telmisartan, metoprolol, etc.). Healthy participants were selected purely based on not taking any medication. Efforts were made to match the control group to the case group by age. Hence, for each case, one control was allotted to compare the effect. Due to the unavailability of similar age groups of controls against the case, younger controls were included in a few instances.
2.5. Sample Collection
Prior permission was obtained from the patients to collect the blood sample. The procedure was well explained to the patient. All the samples were collected in fasting conditions (8-12 hours).
2ml of blood was collected in Ethylene Diamine Tetra Acetic Acid (EDTA) tubes to measure the Biochemical parameter HbA1c. 3ml of blood was collected into a fluoride tube with anticoagulant to measure biochemical parameters, including fasting blood sugar. A sample of 4ml of human venous blood was collected in a citrate vial for the isolation of PBMCs.
2.6. Biochemical Investigation
FBS was measured using a blood sample collected in a fluoride tube through a Fully Automated EM-200 XL System Pack based on the principle of photometry, and it was estimated by the Glucose Oxidase Peroxidase (GOD-POD) method [19].
HbA1c was measured by a blood sample collected in an EDTA tube using an Alere Affinion™ AS100 Analyzer/ Affinion™ 2 Analyzer based on the principle of Chromatography, and it was estimated via the Boronate Affinity Chromatography (BAC) method [20].
2.7. Materials
Curcuma longa, Elettaria cardamomum, Piper longum, curry leaves (Murraya koenigii), Histopaque® (Himedia, density gradient 1.077 grams/ mL), Dulbecco′s Phosphate Buffered Saline (Himedia), RPMI 1640 (Himedia, with L-Glutamine, 25mM HEPES buffer and sodium bicarbonate), antibiotic-antimycotic (penicillin and streptomycin Himedia), Fetal Bovine Serum (Himedia), MTT kit (ABCAM US), distilled water, 96 well plate (non-adherent), and Trypan blue dye.
2.8. Methods
2.8.1. Herbal Extraction, Green Synthesis of MgONPs, and Characterisation TURMERIC, CARDAMOM, and PIPPALI
As per our published literature, the herbal extraction was performed using a Soxhlet apparatus and characterized by HPTLC [17]. The green synthesis of MgONPs was obtained using curry leaves. FTIR, DLS, and zeta size analysis were performed for MgONPs, and the size was found to be 55nm. (as reported in a study by Insha et al., 2024) [17]. Additional FTIR analysis was performed for the herbal combination, Curc + Card + Pippali, and the data are presented in this paper for the first time.
2.8.2. Sample Preparation
Trial solutions or sample solutions were prepared based on the alkaloid percentages established in herboceuticals. The samples were prepared in a 1:2:3 ratio of Cardamom, Pippali, and curcuma, respectively, to achieve a working concentration of 5mg/ml. Similarly, 5mg/ ml MgONPs were dissolved in the working volumes of 5mg/ ml of Cur + Card + Pip, and the final stock was made up to 10mg/ ml.
2.8.3. Fourier Transform Infrared (FTIR) Spectroscopy
FTIR was used to examine the surface functional groups of MgONPs using the Bruker ALPHA II FTIR Spectrometer. It was carried out to examine the formation of magnesium oxide nanoparticles mediated by the functional groups in curry leaves (Murraya koenigii). The range was preserved at a resolution of 4 cm−1, between 4000 and 500 cm−1, with an atmospheric compensation setting. The result spectrum was checked in transmittance mode.
2.8.4. Isolation of PBMCs
A 4 ml human venous blood sample was placed in citrate vials. The samples were mixed thoroughly, and an equal amount of DPBS was added to the blood. The blood was gently layered on top of the Ficoll Histopaque. The tubes were centrifuged for 30 minutes at 400 x g in a swing-out bucket (REMI). PBMCs formed in the interphase between Histopaque and medium were removed carefully, followed by washing (centrifuged at 400 x g for 10 minutes) twice with 10 ml of DPBS. The cells were resuspended in RPMI-1640 medium. Cell counting was performed in a Neubauer’s chamber and checked for viability using Trypan blue dye. Cell viability was evaluated by counting the absolute numbers of viable and non-viable cells and the percentage of viable cells over non-viable cells. All cell viabilities were greater than 95%.
2.8.5. MTT Assay
The cells were seeded in a non-adherent 96-well flat-bottom microtiter plate at a density of 5 × 103 cells/ well. They were treated with 10 µl of 500 µg/ ml, 400 µg/ ml, 300 µg/ ml, 200 µg/ ml, and 100 µg/ ml of the desired herbal extract (Cur + Car + Pip) conjugated with and without NPs for 24 and 48 hours at 37 °C in a CO2 incubator. 5 mg/ ml MTT was added to each well. The plate was incubated for 4 hours at 37°C in a CO2 incubator. Subsequently, 120 μl of solvent (provided in the kit containing 10% sodium dodecyl sulfate in 0.001 N hydrochloric acid) was added for dissolution of the crystals. The plate was quantified using the Synergy HTX multimode microplate reader from BioTek at 540 nm. The relative cell viability (%) in the sample-treated wells for the control wells was estimated by (Fig. 1):
 |
(1) |
Where Blank1 = Media + Treatment
Blank2 = Media alone
2.9. Statistical Analysis
Data entry was performed using Microsoft Excel 2021. Descriptive and frequency statistics were used to present the data for categorical variables. A two-sample independent t-test was conducted to compare the mean values between the FBS and HbA1C groups using Open Epi software. Quantitative variables were presented as mean ± SD. The data were analyzed with the Statistical Package for the Social Sciences (SPSS), version 26.0. For statistical analysis, the Mann-Whitney and Wilcoxon signed-rank tests were used to compare p-values for evaluating significance between subgroups. If p is greater than 0.05, it indicates that the results are not statistically significant.
3. RESULTS AND DISCUSSION
The study comprises 20 participants, including 10 normoglycemic and 10 T2DM patients, who satisfied the inclusion and exclusion criteria. The demographic information, such as age, gender, and duration (in the case of T2DM), was taken from participants, and the results of FBS and HBA1C are mentioned in Tables 1 and 2.
3.1. HPTLC oF Herbal Extracts
As per our published article (along with already published data in a study by Khan et al., 2024), the hydroethanolic extract of turmeric (Curcuma longa) showed the presence of curcuminoids, flavonoids, and 3-butylligusticumlactone. The cardamom showed a single prominent peak with 100% saturation of phytochemical terpenoids, including contents like myrcene, cineole, linalool, and beta-caryophyllene, at a concentration of 5mg/ ml. The Pippali extract revealed the presence of isopiperine and piperine [17].
The active compound of turmeric, ‘curcuminoid,’ has been extensively studied for its potential therapeutic effects, particularly in diabetes and CVDs. [21]. Another plant is cardamom, a spice known for its culinary use and medicinal properties [22].
To enhance the bioavailability of turmeric and cardamom, Pippali herb was introduced in a fixed ratio according to herboceutical principles. Pippali and its active compound ‘piperine’ play a significant role as bio-enhancers by increasing the bioavailability and efficacy of various drugs and nutrients. This is achieved through inhibiting metabolic enzymes, enhancing intestinal permeability, and inhibiting efflux pumps [23]. Pippali is often combined with turmeric and cardamom in traditional medicinal formulations for several synergistic reasons, as both plants have significant medicinal properties that complement each other, enhancing the overall therapeutic efficacy [24]. Curcumin is a potent antioxidant that can neutralize free radicals and reduce oxidative stress, a significant factor in CVDs [25]. Cardamom contains compounds such as cineole and limonene, which have shown anti-inflammatory and antiplatelet effects [26].
3.2. FTIR of Herbal Extraction
The FTIR spectra analysis was performed in the range of 500-4000cm-1. The strong infrared bands near 3922.99 cm-1, 3361.42cm-1, and 3265.49cm-1 were observed for the O–H bond vibrations of the hydroxy group.
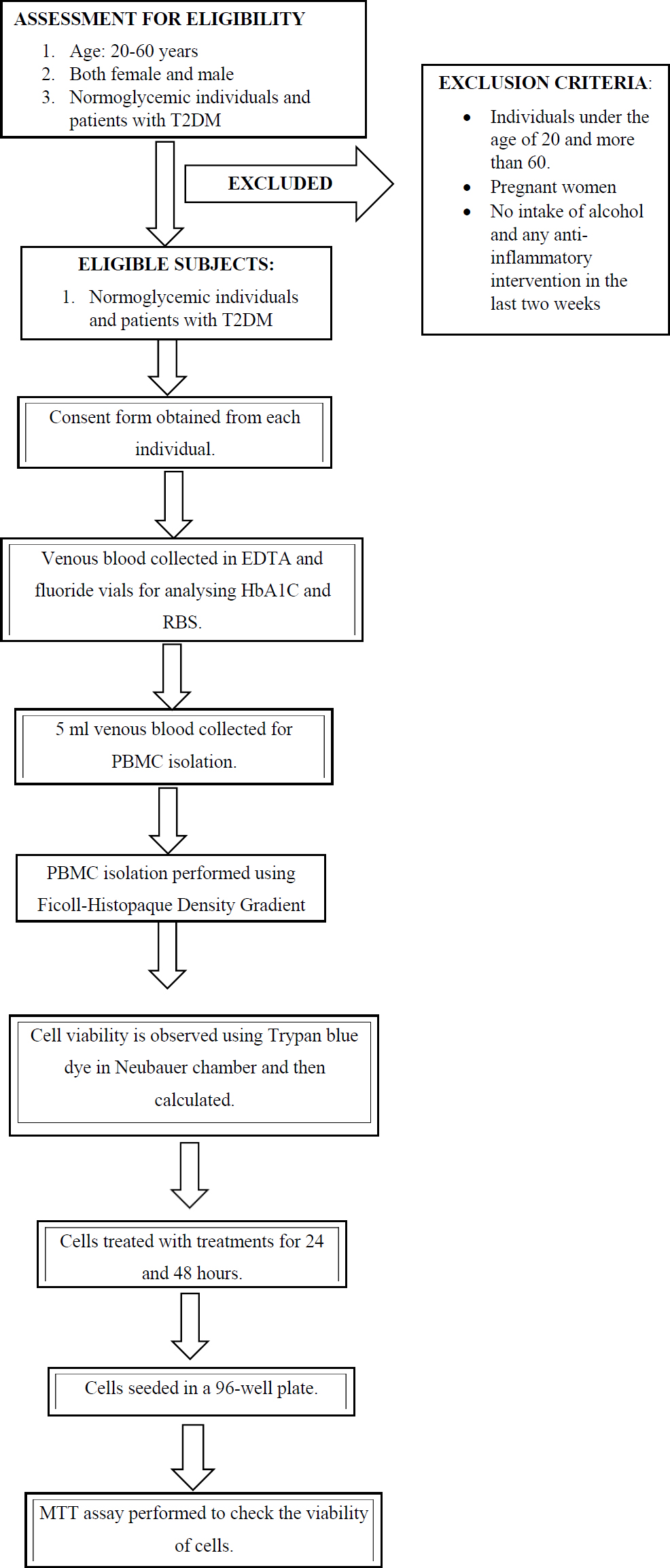
Schematic flow of the methodology.
| Normoglycemic | ||||||
|---|---|---|---|---|---|---|
| S. No | Healthy Volunteer | Age | Gender | Duration of T2DM | FBS (mg/dl) | HbA1C (%) |
| 1 | Healthy Volunteer 1 | 28 | Female | No | 98 | 5.11 |
| 2 | Healthy Volunteer 2 | 35 | Male | No | 88 | 4.72 |
| 3 | Healthy Volunteer 3 | 27 | Male | No | 80 | 4.75 |
| 4 | Healthy Volunteer 4 | 42 | Male | No | 94 | 5.15 |
| 5 | Healthy Volunteer 5 | 50 | Female | No | 91 | 4.93 |
| 6 | Healthy Volunteer 6 | 45 | Female | No | 104 | 5.25 |
| 7 | Healthy Volunteer 7 | 51 | Female | No | 105 | 5.33 |
| 8 | Healthy Volunteer 8 | 38 | Male | No | 80 | 5.09 |
| 9 | Healthy Volunteer 9 | 25 | Male | No | 98 | 4.84 |
| 10 | Healthy Volunteer 10 | 23 | Female | No | 78 | 4.80 |
| T2DM | ||||||
| S. No | Patient | Age | Gender | Duration of T2DM | FBS (mg/dl) | HbA1C (%) |
| 1 | Patient 1 | 28 | Female | 3 years | 149 | 9.65 |
| 2 | Patient 2 | 32 | Male | 2 years | 152 | 9.06 |
| 3 | Patient 3 | 26 | Male | 2 years | 298 | 10.47 |
| 4 | Patient 4 | 38 | Male | 5 years | 378 | >15 |
| 5 | Patient 5 | 52 | Female | 5 years | 149 | 9.65 |
| 6 | Patient 6 | 45 | Female | 3 years | 148 | 9.62 |
| 7 | Patient 7 | 48 | Female | 4 years | 149 | 8.65 |
| 8 | Patient 8 | 53 | Male | 8 years | 260 | 9.75 |
| 9 | Patient 9 | 51 | Male | 7 years | 136 | 9.31 |
| 10 | Patient 10 | 57 | Female | 8 years | 203 | 10.08 |
| - | (N=20) | Statistics t-value | p-value | |
|---|---|---|---|---|
| NG (n=10) | T2DM (n=10) | |||
| FBS MG/DL (MEAN ± SD) | 91.6±9.93 | 200.5.4±84.21 | -4.06 | 0.002** |
| (HBA1C%) (MEAN ± SD) | 4.99±0.21 | 10.14±1.78 | -9.08 | 0.000001*** |
| AGE (MEAN ± SD) | 36.4±10.41 | 43.0±11.20 | -1.36 | 0.189* |
The peak at 2982.57cm-1 was observed for the -CH- and -CH2-CH3 bond vibrations. At the peak, 1641.73cm-1 and 1416.23cm-1 is related to C=C and C-O bond vibrations, respectively. The peak at 1045.11cm-1 and 877.46cm-1 was observed for the -C-H bond vibrations (Fig. 2).
3.3. Characterisation of MgONPs
3.3.1. Dynamic Light Scattering (DLS) and Zeta Potential (ZP)
According to our published article, the particle size was determined to be 54.95 nm. The Zeta Potential (ZP) reflects the stability of nanoparticles, as it directly indicates their surface charge, and was measured at -0.87 mV [17]. Numerous studies have demonstrated that metal nanoparticles in the size range of 10-100 nm can efficiently enter and interact with Peripheral Blood Mononuclear Cells (PBMCs) [27].
Nanoparticles within this size range are typically regarded as ideal for drug delivery, as they facilitate Enhanced Permeability and Retention (EPR) effects. Particles larger than 100 nm are more likely to be cleared from the bloodstream by sphagocytes [28].
The green synthesis of NPs often results in biocompatible and less toxic materials [29]. In this study, the use of curry leaves to synthesize MgONPs represents a green and innovative approach to enhancing the medicinal activity of herbal formulations. When used as an adjuvant to turmeric and Pippali, MgONPs can significantly boost their therapeutic properties. [30] Additionally, curry leaves have hypoglycemic properties that help manage blood glucose levels. They are rich in antioxidants, which help in reducing oxidative stress [31]. Moreover, curry leaves contain various bioactive compounds, such as alkaloids, flavonoids, and phenolic acids, that act as reducing and stabilizing agents in the green synthesis of MgONPs [32, 33].

FTIR spectrum of herbal extraction.
Among metal oxide NPs, MgONPs are biocompatible and suitable for biomedical applications, including drug delivery. It can provide a controlled release mechanism, ensuring a sustained therapeutic effect and maintaining consistent blood levels of the active compounds. The conjugation of MgONPs was thought to be beneficial to diabetic patients in several ways. The study claims that magnesium supplementation for diabetic cells improves the reversibility of insulin resistance. To reverse insulin resistance, it is thought that Magnesium Oxide (MgO) nanoparticles could be molecularly modified to provide improved therapeutic efficacy [34-37]. A study demonstrated that curcumin-loaded MgONPs and solid lipid nanoparticles effectively attenuated aluminum-induced neurotoxicity in albino rats, suggesting that MgONPs can serve as carriers for sustained and targeted delivery of curcumin [38]. Another study synthesized MgONPs via a sol-gel reaction and investigated their potential as carriers to deliver Mg2+ to affected joints in osteoarthritis treatment. The findings indicated that MgONPs could prolong the Mg2+ release time from 0.5 hours to 12 hours, demonstrating their capability for sustained delivery [39].
Another study suggests that MgONPs can enter cells via endocytosis, a process where the cell membrane engulfs external particles, forming vesicles that internalize the nanoparticles [29].
These studies collectively highlight the potential of MgONPs to modulate the release kinetics of herbal compounds, thereby improving their pharmacodynamic profiles.
MgONPs were conjugated with the herbs, Cur + Car + Pip, and further evaluated for cytotoxicity using the MTT assay, which was conjugated with herbal extracts and subjected to a cytotoxicity assay by using PBMCs. For MTT assay in all tests, the untreated cells of NG and T2DM individuals were evaluated as the control. Optical densities were measured at 540nm.
3.3.2. PBMC Viability After Treatment With Extracts
Recently, our Laboratory documented for the first time a comparison of cytotoxicities between two different herbal combinations with and without NPs using PBMCs as the primary cell line involved in immune response and inflammatory response. It was isolated from type 2 diabetic patients. PBMCs from normoglycemic individuals served as the control. The combinations were Curcuma + Pippali with and without MgONPs, and Cardamom + Pippali with and without MgONPs. The treatments were given as per this: 25µg/ 5µL, 60µg/ 15µL, 125µg/ 25µL, 250µg/ 50µL, and 375µg/ 75µL [17]. The effect of hydroethanolic solvent on cell viability was found to be negligible at all the volumes, i.e., it was found to be greater than 90% (p >0.05). All the data were represented as mean values ± SEM. It was quite interesting to observe that the viability of Curcuma + Pippali, when treated in normoglycemic cells for 24 hours, was found to be 79.83 ± 1.2% for the lowest dose (25 µg) and 7.93 ± 0.6% at the dose of 375 µg. Whereas, in the case of Curcuma + Pippali + MgONPs, the values were 74.50 ± 0.9% and 6.47 ± 1.1%, which are not significantly different from each other. Within 48 hours, the values for the same treatment on the NG declined to 49.50 ± 2.0% and 4.75 ± 0.5%. When the same treatment without NPs was observed in T2DM patients, the values for 24 and 48 hours were found to be 76.13 ± 0.8% and 6.98 ± 0.7% for the doses mentioned as 25 µg and 375 µg, respectively. All data are represented as mean values ± SEM.
The same doses were checked for the combination of Cardamom and Pippali with and without NPs under the same conditions. The values were found to be 95.12 ± 1.3% and 42.77 ± 2.0% for the 24-hour NG group, whereas in the case of Cardamom + Pippali + MgONPs, the values were 91.23 ± 1.29% and 37.50 ± 2.9%. In the same way for the T2DM group, results were observed as 92.87 ± 2.31% and 35.50 ± 2.76% for the same treatment without NPs and 90.45 ± 1.39% and 32.25 ± 2.13% with Cardamom + Pippali + MgONPs for 24 hours and 48 hours the results were 74.40 ± 3.51% and 9.90 ± 1.13% without NPs. Whereas, with NPs, the values were 68.46 ± 0.86% and 7.98 ± 1.68% for the lowest and highest concentrations, respectively. There was a remarkable difference in the viabilities of both groups, i.e., Curcuma and Cardamom, whether it was conjugated with MgONPs or not. The Cardamom + Pippali with and without NPs showed minimal cytotoxicity in both cases, i.e., NG and T2DM, as compared to Curcuma (p>0.001) [17].
Knowing the medicinal values of curcuma, it was thought to combine all three herbs and recheck their cytotoxicity with the hypothesis that the presence of cardamom can mitigate the lower cell viability due to the treatment with Curcuma. Anticipating the trio with MgONPs (Cur + Card + Pip + NPs) can be a successful combination for future research. Hence, it was important to check cell viability beforehand using the MTT assay.
PBMC viability after treatment with 10 µL of Cur + Card + Pip and Cur + Card + Pip + NPs in the range of 100-500 µg/ ml is shown in Fig. (3a-b) for both the NG and T2DM groups at 24 and 48 hours, respectively.
3.3.3. Cell Viability Evaluation Post 24 Hours
The cell viabilities were observed at 24 hours to be 96.45 ± 0.003% and 92.75 ± 0.026%, respectively, for the combination Cur + Card + Pip alone and Cur + Card + Pip + MgONPs at 100 µg/ ml in NG (p >0.01). While in the case of T2DM, 93.63 ± 0.01% and 90.82 ± 0.018% were comparable to that of NG-24 hours at the same treatment, i.e., 100µg/ ml with and without MgONPs.

MTT assay was performed on PBMCs for NG individuals and T2DM patients when treated with concentrations (100-500 µg/ ml) with and without MgONPs. (a) Cytotoxicity of Curc + Card + Pip in the case of NG and T2DM after 24 hours, with and without NPs. (b) Cytotoxicity of the same treatments under the same conditions after 48 hours. Viabilities are expressed as mean values.
Cytotoxicity increased at 400-500 µg/ ml for both NG and T2DM. The viabilities at 500 µg/ ml with and without NPs in the case of NGs are as follows: 40.86 ± 0.01%, 38.03 ± 0.01%, 37.20 ± 0.01%, and 36.02 ± 0.01%.
3.3.4. Cell Viability Evaluation Post 48 Hours
The values at 48 hours were 73.62 ± 0.15% and 62.02 ± 0.046% for the respective doses in NGs. In the case of T2DM, the values were 68.41 ± 0.01% and 59.44 ± 0.01%, respectively, after the treatments with and without NPs (p >0.01). At a treatment of 500µg/ ml, 21.19 ± 0.01% and 11.08 ± 0.01% were the obtained cell viabilities for the NG group. For T2DM, the values were found to be 12.73 ± 0.01% and 9.30 ± 0.004%, respectively, for the with and without NPs groups.
There was not a significant difference in the viability of PBMCs between the NG and T2DM groups, especially when treated with their respective NPs, either at 24 or 48 hours (p > 0.05). Even in the case of NG, the 24- and 48-hour comparisons revealed that all treatments were highly significant, except for the treatment at 300 µg/ ml (p = 0.765, p > 0.05). Conversely, in T2DM cases, the values did not demonstrate a significant pattern as the doses increased (p > 0.05). The conjugation of NPs did not affect the viability in both NG and T2DM cases, as most treatments were significant. (p < 0.05).
The vehicle (hydro ethanol) showed minimal effect on PBMCs viability (p < 0.05), as it was found to be < 95% viability at the volume of 10µL in the PBMCs. Notably, a significant difference was observed in the cell viabilities at 24 and 48 hours, irrespective of NG and T2DM cases with and without NPs (p < 0.05).
The CC50 value (cytotoxic concentration 50%) is a measure of the concentration of a drug or compound that is cytotoxic to 50% of a population of cells, CC50 values were determined by plotting the concentration of extract (100-500 µg/ ml) on the x-axis against the percentage of viability on the y-axis with dose-response curves. Table 2 contains the CC50 data for two categories of plant extracts, i.e., with and without NPs at 24 and 48 hours.
| Treatments | Assay Conditions | CC50 (µg/ ml) |
|---|---|---|
| Cur + Car + Pip | 24 hours NG | 452 |
| 48 hours NG | 300 | |
| 24 hours T2DM | 430 | |
| 48 hours T2DM | 240 | |
| Cur + Card + Pippali NP | 24 hours NG | 410 |
| 48 hours NG | 330 | |
| 24 hours T2DM | 425 | |
| 48 hours T2DM | 240 |
The CC50 for Cur + Card + Pip with and without NPs was 452 and 410 µg/ ml, respectively, in 24 hours, whereas in T2DM patients, the same treatments with and without NPs were found to have CC50s of 300 and 330 µg/ ml, respectively. The values were the same in the case of T2DM (48 hours), regardless of whether it was with or without NPs (CC50 = 240 µg/ ml) (Table 3).
Unlike the test carried out earlier, which revealed better results with Cardamom + Pippali with and without NPs, combining all three surpassed the lower viability due to the presence of Curcuma alone.
The NPs were well tolerated by the PBMCs as evidenced by the cell viability results. As in most of the concentrations, the statistical difference between with and without Nps was non-significant or mildly significant. (p>0.05). The incorporation of the MgONPs in the herbal extract probably facilitates the entry of drugs into cells due to the differences in electrical charge to the cell membrane, hence enhancing the stability [40]. Another study claims that the acquired Abrus precatorius bark-produced MgONPs were evaluated for toxicity using zebrafish embryos as a model organism. The results showed that the MgONPs were harmless [41].
This study indicates that there was no significant difference in PBMC viability between the NG and T2DM groups, particularly when treated with Curc + Card + Pip with and without NPs and at both 24 and 48-hour intervals (p > 0.05). It can be potentially utilized for future research related to Cardioprotective properties (Table 4).
A) NG vs PT¥.
| S.No | Criteria |
Treatment 1 (p-value) |
Treatment 2 (p-value) |
Treatment 3 (p-value) |
Treatment 4 (p-value) |
Treatment 5 (p-value) |
|---|---|---|---|---|---|---|
| 1) | NG vs. T2DM in 24 hours | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 2) | NG vs. T2DM in 48 hours | <0.0001 | <0.0001 | 0.296* | 0.002 | <0.0001 |
| S.No | Criteria |
Treatment 1 (p-value) |
Treatment 2 (p-value) |
Treatment 3 (p-value) |
Treatment 4 (p-value) |
Treatment 5 (p-value) |
|---|---|---|---|---|---|---|
| 1) | 24 vs. 48 hours in NG | <0.0001 | <0.0001 | 0.765* | <0.0001 | <0.0001 |
| 2) | 24 vs. 48 hours in T2DM | <0.0001 | 0.126* | 0.179* | 0.062* | 0.012 |
| S.No | Criteria |
Treatment 1 (p-value) |
Treatment 2 (p-value) |
Treatment 3 (p-value) |
Treatment 4 (p-value) |
Treatment 5 (p-value) |
|---|---|---|---|---|---|---|
| 1) | With vs. without in 24 hours | <0.0001 | <0.0001 | 0.025 | 0.422* | 0.019 |
| 2) | With vs. without in 48 hours | 0.004 | 0 .001 | <0.0001 | <0.0001 | <0.0001 |
4. LIMITATIONS
However, this was a small-scale pilot study, and its primary limitation was the small sample size. The previous study was volume-dependent, comparing the cytotoxicities of Curcuma and Cardamom (Khan et al., 2024) [2]. To make a valid comparison between both situations, a volume-dependent cytotoxicity analysis is necessary. To get a rough estimation, this study can be compared to the previous one. Additionally, comparing the same age group of healthy controls and T2DM patients can be challenging, as Type 2 diabetes accounts for over 90% of all diabetes cases in India. According to the Indian Council of Medical Research – India Diabetes (ICMR INDIA) study published in 2023, the prevalence of diabetes is 10.1 crores [42]. Middle-aged individuals have a significantly higher prevalence of Type 2 diabetes compared to younger and older individuals [43].
CONCLUSION
Medicinal plants have long been integral to traditional medicine, and emerging therapies show promise in further reducing cardiovascular risks in this population. Taking a holistic approach to these issues can significantly enhance outcomes for individuals with diabetes. This study is the first to combine three herbs for their synergistic effects, conjugated with NPs. Previous research demonstrated the efficacy of Cardamom + Pippali and Cardamom + Pippali + MgONPs using the MTT assay on PBMCs from T2DM patients, comparing them to normoglycemic healthy individuals. However, given the well-documented antiplatelet and anti-inflammatory properties of Curcuma, it was decided to combine all three herbs and conjugate them with MgONPs to evaluate their cytotoxicity. This study presents the cytotoxic evaluation of the combination of Curcuma, Cardamom, and Pippali, both with and without NPs, at concentrations ranging from 100 to 500 µg/ ml. The CC50 for Cur + Card + Pip without and with NPs was > 400 µg/ ml after 24 hours for both the NG and T2DM groups. After 48 hours, the concentrations decreased to ≥ 300 µg/ ml for the NG group, with and without NPs, while the CC50 in T2DM remained at 240 µg/ ml in both treatments, with and without NPs. Therefore, the appropriate dosages can be determined accordingly in future research focusing on their anti-inflammatory, antioxidant, and antiplatelet activities.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: N.P.: Study conception and design; I.K.: Data collection; R.V.: Data analysis and interpretation; P.M.: Methodology. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| T2DM | = Type 2 Diabetes Mellitus |
| NG | = Normoglycemic |
| PBMCs | = Peripheral Blood Mononuclear Cells |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Ethics Committee / Independent Ethics Committee PIAR - Dr Umesh Yelne, Dr Vaishali Deshapande, Dr Arunraj GR IECHR Ref: PIAR/2023/06 /IEC-PIAR HR.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
All the data and supporting information are provided within the article.
FUNDING
This study was funded by RDC (Research and Development Centre), Parul University, Vadodara, Gujarat, India, (RDC/IMSL/137).
ACKNOWLEDGEMENTS
Declared none.


