All published articles of this journal are available on ScienceDirect.
Recent Advances in Synthetic Approaches for 1,3,4-Oxadiazole Derivatives: A Comprehensive Review on Therapeutic Applications
Abstract
1,3,4-Oxadiazoles are a vital class of nitrogen-containing five-membered heterocycles that have drawn considerable attention due to their diverse pharmacological properties. These compounds exhibit a broad spectrum of biological activities, including anticancer, antibacterial, anti-inflammatory, anti-HIV, anti-tubercular, anti-diabetic, and antifungal effects. Their unique structural framework makes them highly valuable in drug discovery and medicinal chemistry. This review provides a comprehensive overview of the various synthetic strategies employed to develop 1,3,4-oxadiazole derivatives. Both conventional and advanced methods are discussed in detail, including the cyclization of hydrazides, oxidative cyclization, and environmentally friendly green chemistry approaches. Additionally, we highlight how structural modifications influence their bioactivity, paving the way for the design of more potent therapeutic agents. Beyond synthesis, this paper explores the pharmacological evaluation of 1,3,4-oxadiazoles through various biological assays. The relationship between molecular structure and biological activity is examined, offering valuable insights into the rational design of novel derivatives with enhanced efficacy and selectivity. Given their versatility as a privileged scaffold in medicinal chemistry, 1,3,4-oxadiazoles hold significant promise in the development of new treatments for various diseases. Overall, this review serves as a valuable resource for researchers interested in the synthesis and therapeutic potential of 1,3,4-oxadiazole derivatives, aiding in the development of innovative drugs with improved pharmacological properties.
1. INTRODUCTION
Heterocyclic compounds are among the most widely studied chemical structures in medicinal chemistry due to their significant role in drug development [1]. These compounds serve as fundamental building blocks in many bioactive molecules, contributing to their therapeutic properties [2]. One such heterocyclic system is oxadiazole, a five-membered ring structure composed of one oxygen atom, two nitrogen atoms, and two double bonds [3]. Due to its unique electronic configuration and stability, oxadiazole has attracted considerable attention in pharmaceutical research [4]. Over the past several years, extensive investigations have been conducted on oxadiazole derivatives, revealing their potential in treating various pathological and pharmacological conditions [5].
Among the five-membered heterocyclic systems, several key structures, such as pyrrole, oxadiazole, thiadiazole, and triazole, have gained prominence due to their biological significance [6]. These heterocyclic scaffolds are found in numerous bioactive compounds, enhancing their medicinal properties [7]. Among these, 1,3,4-oxadiazole is of particular interest to researchers due to its versatile pharmacological activities [8]. The presence of the oxadiazole core in various drug candidates has been linked to improved biological efficacy, making it an essential framework in medicinal chemistry [9].
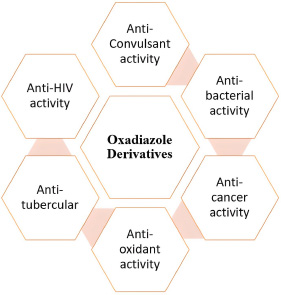
Scientific studies have highlighted the broad spectrum of biological activities exhibited by 1,3,4-oxadiazole derivatives [10]. These compounds have demonstrated strong antimicrobial properties, making them effective against a variety of bacterial and fungal infections [11]. Additionally, their antitubercular activity suggests potential applications in tuberculosis treatment [12]. The vasodilatory effects of these molecules contribute to their role in cardiovascular therapies, while their cytotoxic and antitumor properties indicate their potential in cancer treatment [13]. Furthermore, 1,3,4-oxadiazole derivatives exhibit analgesic and anti-inflammatory effects, making them valuable for pain management and inflammatory conditions [14]. Other pharmacological activities, such as hypolipidemic and ulcerogenic properties, further expand their therapeutic potential [15].
Due to their diverse biological activities and structural flexibility, 1,3,4-oxadiazole derivatives continue to be an area of active research in drug discovery [16]. Their ability to interact with various biological targets makes them promising candidates for the development of novel therapeutic agents [17]. As a result, these compounds hold significant potential in the pharmaceutical industry, paving the way for the discovery of new and effective treatments for various diseases (Fig. 1) [18].
1.1. Chemistry of Oxadiazole
Heterocyclic drugs like 1,3,4 oxadiazole are made up of five members, one oxygen and two nitrogen atoms [19]. Substitution of two nitrogen (-N=) groups of the pyridine type for two -CH= groups is considered to be the source of furan [20]. Oxadiazole has four possible isomers: 1.3,4-Oxadiazole (1), 1,2,4-Oxadiazole (2), 1,2,3-Oxadiazole (3), and 1,2,5 Oxadiazole (4) (Fig. 2) [21, 22].
Chemistry of oxadiazole analogues: current status and applications.

Isomeric structures of 1,3,4-oxadaizole unit.
Unsubstituted 1,3,4-oxadiazole, among all the isomeric oxadiazoles, possesses the more reliable isomeric structure [23]. A liquid having a 150 °C boiling point is 1,3,4-oxadiazole's basic building block. Lower alkyl derivatives can also contain liquids. When 1,3,4-Oxadiazole has two methyl groups, it is entirely soluble in water; aryl substituents significantly decrease the solubility [24].
On the market, oxadiazole derivatives include Furamizole, Nosapidil, and Tiodazosin11. This review's objective is to give a summary of some of the pharmacological actions of 1,3,4-oxadiazole that is 2,5-disubstituted [25].
2. VARIOUS ROUTES FOR THE SYNTHESIS OF 1,3,4-OXADIAZOLE DERIVATIVES
2.1. Synthesis of 5-Aryl-2-amino-1,3,4-oxadiazole (Compound 1) by Patel and Patel (2010-2012)
2.1.1. Methodology
Patel and Patel have described the synthesis of 5-aryl-2-amino-1,3,4-oxadiazole (Compound 1) in good yield (62–70%) by treating arylamine with carboxylic acid derivatives in the presence of a dehydrating agent, e.g., thionyl chloride or phosphorus oxychloride [26-28]. The reaction is conducted under controlled conditions of temperature and time to facilitate the formation of the oxadiazole ring. The product is recrystallized and analyzed by methods such as TLC, IR spectroscopy, and determination of melting point.
2.1.2. Reaction Conditions
- Reagents: Arylamine, carboxylic acid derivatives, dehydrating agent (e.g., thionyl chloride, phosphorus oxychloride)
- Solvent: [Specify solvent, if mentioned]
- Temperature: [Provide specific temperature, if available]
- Reaction Time: [Provide specific time, if available]
- Yield: 62%–70%
- Characterization: TLC, IR, melting point analysis
- Reference: Patel and Patel [26-28].
2.2. Synthesis of an Innovative Class of 1,3,4-Oxadiazoles by Kerimov et al. (2020)
2.2.1. Methodology
Kerimov reported synthesis of a new series of 1,3,4-oxadiazoles by the reaction of arylhydrazines with acid chlorides under mild conditions with DMF or DMSO as solvent and triethylamine as base [29]. The reaction was carried out at a temperature of [provide temperature if available] for [reaction time]. Upon completion, the reaction mixture was purified by column chromatography, and the product was identified by TLC and IR spectroscopy. The yield of the target product was between 33% and 60%, based on the substituent groups on the aromatic rings (Scheme 1).
2.2.2. Reaction Conditions
- Reagents: Arylhydrazine, acid chlorides, triethylamine
- Solvent: DMF or DMSO
- Temperature: 60–70°C
- Reaction Time: 6 hours
- Yield: 33%–60%
- Characterization: Column chromatography, TLC, IR spectroscopy
- Reference: Kerimov et al. [29].
2.3. Bicyclodesulfurization of N-acyl-thiosemicarbazide to 2-Amino-1,3,4-Oxadiazole (Scheme 2)
2.3.2. Procedure
(a) Reaction Setup: Combine N-acyl-thiosemicarbazide (4) and thiosemicarbazide (5) in a suitable solvent (e.g., DMF).
(b) Addition of Reagent: Add 1.5 equivalents of EDCI to activate the coupling reaction.
(c) Reaction Conditions: Stir the mixture at room temperature for 4–8 hours, monitored by TLC.
(d) Work-up and Purification: Extract with ethyl acetate, dry, and concentrate. Purify by recrystallization.
(e) Yield: The reaction typically yields 2-amino-1,3,4-oxadiazoles (6) in 65–90% yield [30, 31].
2.3.3. Characterization
Confirm product via IR, NMR, and mass spectrometry. The synthesis of 2,5-disubstituted-1,3,4-oxadiazole (9) involves a condensation reaction between aldehydes and acyl hydrazines, followed by sodium bisulfate-mediated oxidative cyclization in an ethanol:water (1:2) solution. This reaction can be performed using either microwave-assisted or conventional heating methods (Scheme 3).
2.3.3.1. Reaction Design
- Condensation: Aldehyde reacts with acyl hydrazine to form an intermediate.
- Oxidative Cyclization: Sodium bisulfate is used as the oxidizing agent in ethanol:water (1:2) solution.
2.3.3.2. Methods
- Microwave-Assisted Method: This method offers higher yields (70–90%) and shorter reaction times.
- Conventional Heating: Requires more time and typically provides comparable yields (70–90%).
2.3.3.3. Comparison of Methods
- Microwave-assisted synthesis yields higher results in a shorter duration compared to conventional heating, as reported by Sangshetti et al. and other studies [32-35].
The overall synthesis shows efficient yields (70–90%), with the microwave method being more time-efficient.
2.4. Synthetic Methodology for 2-Amino-1,3,4-oxadiazoles
2.4.1. Primary Synthetic Route
- The most common method involves the cyclization of 2-acyl-hydrazinecarbothioamides (12) in the presence of a coupling agent, forming 2-amino-1,3,4-oxadiazoles (11).
2.4.2. Intermediate Preparation
- The intermediate (compound 4) is synthesized through a multi-step sequence, starting from carboxylic acids or esters (with R1 groups) and ending with isothiocyanates or isocyanates (with R2 groups).
2.5. Study Design and Methodology for the Synthesis of 1,3,4-Oxadiazol-2-amines
2.5.1. Objective
To explore and compare conventional and alternative synthetic approaches for the preparation of 1,3,4-oxadiazol-2-amines.
2.5.2. Conventional Method (Compound 14 – Scheme 5)
- Starting materials: Substituted hydrazides.
- Reaction type: Cyclodehydration.
- Reagents: Typically, POCl3 or SOCl2 used as dehydrating agents.
- Conditions: Reflux temperature for several hours.
- Outcome: Formation of 1,3,4-oxadiazol-2-amines in good yields.
2.5.3. Alternative Method (Compounds 15 → 16 – Entry b, Scheme 5)
- Starting materials: Semicarbazones.
- Reaction type: Electrocyclization.
- Conditions: Thermal or mild acidic/basic conditions (depending on substrate).
- Outcome: Formation of 1,3,4-oxadiazole derivatives with moderate to high yields (60–88%) [38].
According to Rivera et al. and colleagues, the oxidizing agent 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) has proven effective for cyclization of acylthiosemicarbazides. In their study, compound 17 underwent successful cyclization to produce 5-aryl-2-amino-1,3,4-oxadiazoles (compound 18), as illustrated in Scheme 6. A key advantage of this method is the use of safe, inexpensive, and commercially available reagents. Moreover, the approach is suitable for large-scale synthesis, especially in cases where other oxidizing agents are not feasible. The reaction provides excellent yields, ranging from 82% to 94%, for the target 5-aryl-2-amino-1,3,4-oxadiazoles [39, 40].

Schematic representation of the synthetic routes.

5-Substituted-2-amino-1,3,4-oxadiazoles by utilizing EDCI as coupling agent.

Synthesis of 2,5-disubstituted-1,3,4-oxadiazoles by two different methods.

Literature method to prepare 2-amino-1,3,4 oxadiazoles.

5-Substituted-1,3,4-oxadiazol-2-amines from the cyclization reaction of semi carbazones.

Synthesis of 5-aryl-2-amino-1,3,4-oxadiazole from acylthiosemicarbazide and 1,3-dibromo-5,5-dimethylhydantion.
2.6. Synthesis of 5-Substituted-1,3,4-Oxadiazole-2-Thiol(Thione)s (Compound 19) (Scheme 7)
2.6.1. Reagents and Conditions
- Carbon disulfide and acylhydrazides (18) are reacted in a basic alcohol solution.
- This reaction results in the formation of 5-substituted-1,3,4-oxadiazole-2-thiol(thiol) derivatives (19).
2.6.2. Post-reaction Treatment
- The reaction mixture is then acidified to yield the desired products.
2.7. Synthesis of 2-Substituted 5-Amino-1,3,4-Oxadiazoles via Acylthiosemicarbazide Cyclization (Scheme 8) [41, 42]
2.7.1. Reagent and Reaction Conditions
- Oxidizing Agent: Iodine (I2) was used as the oxidizing agent in the acylthiosemicarbazide cyclization.
- Solvent: Ethanol (EtOH) was employed as the solvent for the reaction.
- Catalyst: Potassium iodide (KI) was added as a catalyst/ oxidant to facilitate the reaction.
2.8. Synthesis of 2,5-Disubstituted-1,3,4-Oxadiazoles (Bostrom et al. (Scheme 9) [43, 44]
2.8.2. Method
The cyclodehydration of diacylhydrazines was carried out under anhydrous conditions using triflic anhydride and triphenylphosphine oxide to form 2,5-disubstituted-1,3,4-oxadiazoles.
2.8.3. Advantages
This method provides a safer alternative to POCl3, reducing the associated hazards.
2.8.4. Yield
The reaction yields ranged from 26% to 96%, depending on the nature of the diacylhydrazine substrates.
2.8.5. Characterization
The synthesized compounds were characterized using Thin Layer Chromatography (TLC), Nuclear Magnetic Resonance (NMR), and Infrared (IR) spectroscopy.
By employing a Dehydration agent: EDC during cyclodehydration of diacylhydrazine 55, Nagendra and coworkers revealed the synthesis of unique dipeptide mimetics that are tethered to 1,3,4-oxadiazole 56 and orthogonally protected, with yields ranging from 70% to 92%. (Scheme 10) [45].

Synthesis of 5-substituted-1,3,4-Oxadiazole-2-thiols.

Synthesis of 1,3,4-oxadiazole-2-ammines from of cyclization reaction of acylthiosemicarbazides with iodine.

Cyclodehydration of diacylhydrazine using triphenylphosphine oxide and triflic anhydride.
2.8.6. Reagents and Conditions
The dehydration agent EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide) was employed during the cyclodehydration of diacylhydrazine 55.
2.8.7. Tethering to 1,3,4-Oxadiazole Scaffold
These mimetics were tethered to the 1,3,4-oxadiazole scaffold (compound 56) and orthogonally protected.
2.8.8. Reaction Yields
The yields of the cyclodehydration reaction ranged from 70% to 92%.
N′-substituted cyclopropane carboxylic acid hydrazides were subjected to a Robinson-Gabriel-type cyclization using triphenylphosphine (PPh3) and tetrahalomethanes (CX4; X = Cl, Br, I) as dehydrating agents. The reactions were typically performed in anhydrous dichloromethane (DCM) or toluene under a nitrogen atmosphere at a temperature of 60°C for 4–12 hours. Reaction progress was monitored by thin-layer chromatography (TLC). Upon completion, the reaction mixture was quenched with water, and the organic layer was extracted, dried, and concentrated (Scheme 11) [46].
The crude product was purified via column chromatography on silica gel. The synthesized 1,3,4-oxadiazole derivatives were characterized by IR, NMR (1H and 13C), and mass spectrometry. The method afforded moderate to high yields (65–89%), depending on the halogen used, with iodoform (CI4) providing the best results due to its higher reactivity (65–89%).
XtalFluor-E (Diethylaminodifluorosulfinium tetrafluoroborate, [Et2NSF2]BF4) is a powerful electrophilic fluorinating and dehydrating reagent, originally developed for safer and more efficient fluorination processes. In 2011, Paul et al. reported its novel application in the cyclodehydration of 1,2-diacylhydrazines to form 1,3,4-oxadiazoles, demonstrating a high-yielding and mild methodology Scheme 12 [47].
XtalFluor-E cyclodehydration of diacylhydrazines affords oxadiazoles in 75–95% yields.

Cyclodehydration reaction of diacylhydrazines using EDC.

Effect of halogens in the formation of 1,3,4-oxadiazoles.

Preparation of 1,3,4-oxadiazoles from 1,2-diacylhydrazines using XtaFluor-E.
(1) Activation Step: XtalFluor-E activates the carbonyl oxygen of the diacylhydrazine, likely forming an intermediate complex via fluorosulfur interaction.
(2) Intramolecular Cyclization: The activated intermediate undergoes intramolecular nucleophilic attack by the adjacent nitrogen atom on the second acyl group, forming the oxadiazole ring.
(3) Elimination: The process eliminates a small molecule (HF or other fragments depending on substrate and reagent equivalents), completing the cyclodehydration.
(4) Reaction Conditions
- Reagents: XtalFluor-E (1.5–2.0 eq.)
- Solvent: Dichloromethane (DCM) or acetonitrile (MeCN)
- Temperature: Room temperature or mild heating (25–40°C)
- Reaction Time: 1–3 hours
- Yield: 75–95%, depending on the substrate's substitution pattern

Synthesis of 1,3,4-oxadiazoles from carboxylic acids and hydrazides using HATU and Burgess reagent.
Li et al. developed a workable method for the formation of 1,3,4-oxadiazoles 30 using carboxylic acids and (Scheme 13) and gives good to excellent yields (70–93%) of 1,3,4-oxadiazoles [47].
The method developed by Li et al. for the synthesis of 1,3,4-oxadiazoles involves a direct cyclization of carboxylic acids with acylhydrazides, which offers a workable and efficient approach under mild conditions [47]. This methodology typically results in good to excellent yields (70–93%) of the desired 1,3,4-oxadiazole derivatives (Scheme 13).
In this approach:
(1) Carboxylic acids are reacted with acylhydrazides (R–CO–NHNH2), which are typically prepared in situ or used directly.
(2) The reaction proceeds through dehydrative cyclization.
(3) A coupling reagent or dehydrating agent such as phosphorus oxychloride (POCl3) or polyphosphoric acid (PPA) is employed to facilitate ring closure.
(4) This leads to the formation of the 1,3,4-oxadiazole ring.
In 2006, using the Deoxo-Fluor reagent derived from the mixture of COOH an benzohydrazide (2.2 equiv), Kangani et al. 68–91% depending on COOH and benzohydrazide used (Scheme 14) [38, 48].
Many Another method used to create 2,5-disubstituted-1,3,4-oxadiazoles 32 was a practical one-pot method that involved breaking down monoarylhydrazides and HMPA's acid chlorides solvent while being heated by microwaves. Process time was short, no extra acid catalyst or dehydrating reagent was required, and yields ranged from good to excellent. (Scheme 15) [49-52].
(a) Starting materials:
- Monoarylhydrazides
- Acid chlorides derived from heteroaryl or aryl carboxylic acids (HMPA derivatives)
(b) Reaction setup:
- The mixture is subjected to microwave irradiation, which significantly reduces reaction time and enhances efficiency.
(c) Activation method:
- The monoarylhydrazide and acid chloride are combined directly in a suitable organic solvent.
- No additional acid catalyst or dehydrating reagent is required.
(d) Reaction mechanism
- Initial formation of an acyl hydrazide intermediate via nucleophilic substitution of the acid chloride by the hydrazide.
- Microwave-induced cyclodehydration occurs in situ to afford the 2,5-disubstituted 1,3,4-oxadiazole core.
Pore et al. developed an efficient one-pot synthesis for unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles (compound 33) using trichloroisocyanuric acid (TCCA) as a mild oxidizing and cyclodehydrating agent [53]. In this method, hydrazides react directly with carboxylic acids at ambient temperature, and TCCA facilitates oxidative cyclodehydration without the need for any additional acid catalysts or dehydrating agents.
The reaction is conducted in a suitable organic solvent (such as dichloromethane or acetonitrile) and proceeds rapidly under mild conditions, offering a practical and environmentally friendly alternative to traditional methods. This strategy yields the desired oxadiazoles in high yields (80–94%), with the major advantages being short reaction times and mild, room-temperature conditions (Scheme 16).
Pardeshi et al. reported a practical and efficient method for synthesizing 1,3,4-disubstituted 2,5-oxadiazoles (compound 35) via the oxidative cyclization of acylhydrazones (compound 34) [54]. The reaction involves treating the acylhydrazone substrates with a combination of 1.8% diazabicycloundecene (DBU) and N-chlorosuccinimide (NCS) under mild conditions. This oxidative system facilitates the formation of the oxadiazole ring efficiently, without the need for harsh reagents or metal catalysts.
The method is carried out at room temperature or slightly elevated temperatures in an organic solvent and is notable for its short reaction times, mild reaction environment, and easy workup procedure. A variety of acylhydrazones were successfully cyclized using this approach, affording the desired products in excellent yields ranging from 82% to 96% (Scheme 17) [54, 55].

Synthesis of 1,3,4-oxadiazoles using Deoxo-Fluor.

Synthesis of 2,5-disubstituted-1,3,4-oxadiazoles using microwave heating.

Synthesis of 1,3,4-oxadiazoles using trichloroisocyanuric acid (TCCA).

Oxidative cyclization of acylhyrazones using N-chlorosuccinimide and 1,8-diazabicyclo- [5.4.0] undec-7-ene (DBU).
Dobrotă et al. developed a straightforward method for synthesizing 2,5-disubstituted 1,3,4-oxadiazoles (compound 37) by employing Dess–Martin periodinane (DMP) as an oxidizing agent [56]. In this approach, N-acylhydrazones (compound 36) were subjected to oxidative cyclization using an excess of DMP under mild, metal-free conditions. The reaction proceeded smoothly at room temperature in an organic solvent, such as dichloromethane, leading to efficient intramolecular cyclodehydration and formation of the oxadiazole ring. This method is particularly notable for its benign reaction environment, ease of execution, and high yields ranging from 76% to 92% (Scheme 18) [56, 57].
Polshettiwar and Varma reported a unique method for synthesizing 1,3,4-oxadiazole derivatives (compound 40) via a microwave-assisted condensation reaction [58].
(a) Reactants:
- The synthesis involved the condensation of benzohydrazide (38) with triethyl orthoalkanates (39) as the primary starting materials.
(b) Catalysts:
- Two solid-supported catalysts were employed to facilitate the reaction:
- Phosphorous pentasulfide supported on alumina (P4S10/Al2O3)
- Nafion® NR50 resin
(c) Reaction Conditions:
- The reaction was conducted under microwave irradiation, which significantly enhanced the reaction rate and efficiency compared to conventional heating.
(d) Yields:
- This method consistently yielded 1,3,4-oxadiazoles in good to excellent yields, ranging from 78% to 90% (Scheme 19) [58-60].
Kudelko and Zieliński developed a synthetic methodology for oxadiazole derivatives (compound 43) [47]:
(a) Starting Materials: Commercially available triethyl orthoesters (42) and cinnamic acid hydrazide (41).
(b) Methodology:
- The reaction is conducted under reflux conditions in ethanol.
- Acid catalysis is used to promote cyclization and formation of the 1,3,4-oxadiazole ring.
(c) Advantages:
- Simple and efficient process.
- Short reaction times.
- High yields (80–92%) of analytically pure products.
(d) Outcome: This method offers an effective and reproducible alternative to more complex synthetic routes, ensuring a valuable addition to the field of oxadiazole synthesis. (Scheme 20) [61, 62].
Ramazani and Rezaei established an innovative technique 45 (Scheme 21) [63, 64].
(a) Study Design Design:
- An innovative synthetic technique was developed by Ramazani and Rezaei (Scheme 21) for the preparation of 1,3,4-oxadiazole derivatives [63, 64].
(b) ReactionCondition:
- The reaction was performed under specific conditions that facilitated the synthesis of high-yield products. The yields were consistently in the range of 82% to 95%, demonstrating the efficiency of the method.
(c) Mechanistic Pathway:
- The reaction mechanism proposed by Ramazani and Rezaei simplifies the typical synthetic routes for 1,3,4-oxadiazole derivatives, offering a more efficient and direct approach to the target compounds.
(d) Reproducibility:
- The detailed methodology and the reported high yields suggest the reproducibility of the technique, ensuring its applicability for future research and synthesis of similar derivatives.
3. FUTURE DIRECTIONS IN DRUG DISCOVERY AND DEVELOPMENT
3.1. Overcoming Drug Resistance
The emergence of multidrug-resistant (MDR) infections presents a significant challenge in modern medicine. To address this issue, researchers must develop novel derivatives with enhanced potency against resistant strains. Structural modifications, hybrid molecules, and innovative mechanisms of action can help combat antimicrobial resistance and improve treatment efficacy.
3.2. Advancing Targeted Cancer Therapy
Structure-Activity Relationship (SAR) studies are crucial for refining the selectivity of anticancer compounds. By optimizing molecular features and integrating specialized delivery systems—such as nanoparticles or antibody-drug conjugates—researchers can enhance drug accumulation in tumor cells while minimizing toxicity to healthy tissues.

Oxidative cyclization of N-acylhydrazones using Dess-Martin periodinane.

Nafion catalyzed 1,3,4 oxadiazoles synthesis.

Reaction of cinnamic acid hydrazide with triethyl orthoesters.

Synthesis of disubstituted 1,3,4- oxadiazoles from four components in a one-pot procedure.
3.3. Developing Multi-target Therapeutics
Complex diseases like neurodegenerative disorders and diabetes require drugs that interact with multiple biological targets. Designing multifunctional agents that modulate various pathways simultaneously can improve therapeutic outcomes, reduce side effects, and offer a more comprehensive treatment strategy.
3.4. Implementing Green Synthesis Approaches
Sustainability in drug development is increasingly important. Adopting eco-friendly synthetic methodologies, such as solvent-free reactions, catalytic processes, and renewable feedstocks, can minimize environmental impact while maintaining efficiency and scalability in pharmaceutical production.
3.5. Exploring Novel Therapeutic Applications
Expanding the scope of drug discovery to new therapeutic areas—including antiviral, neuroprotective, and cardiovascular applications—can address unmet medical needs. Investigating the potential of bioactive molecules in diverse disease models may lead to breakthroughs in previously underexplored treatment domains.
3.6. Enhancing Pharmacokinetic and Safety Profiles
Improving drug bioavailability and reducing toxicity are critical for clinical success. Prodrug strategies, nanocarriers, and molecular modifications can enhance absorption, distribution, metabolism, and excretion (ADME) properties, ensuring safer and more effective therapeutic agents.
3.7. Leveraging AI and Computational Tools
Artificial intelligence (AI) and machine learning are revolutionizing drug discovery by enabling predictive modeling, virtual screening, and de novo drug design. These technologies accelerate lead identification, optimize molecular structures, and refine pharmacological predictions, significantly reducing development timelines.
3.8. Encouraging Interdisciplinary Collaboration
Effective drug development requires synergy between medicinal chemists, biologists, clinicians, and computational scientists. Interdisciplinary research fosters innovation, facilitates translational studies, and bridges the gap between preclinical findings and real-world therapeutic applications.
3.9. Navigating Regulatory Pathways for Clinical Translation
Streamlining regulatory approval processes is crucial for the timely introduction of new therapies. Understanding and adhering to global regulatory frameworks, such as those set by the FDA and EMA, ensures compliance with safety and efficacy standards, expediting clinical trials and market entry.
3.10. Encouraging Interdisciplinary Collaboration
Effective drug development requires synergy between medicinal chemists, biologists, clinicians, and computational scientists. Interdisciplinary research fosters innovation, facilitates translational studies, and bridges the gap between preclinical findings and real-world therapeutic applications.
3.11. Navigating Regulatory Pathways for Clinical Translation
Streamlining regulatory approval processes is crucial for the timely introduction of new therapies. Understanding and adhering to global regulatory frameworks, such as those set by the FDA and EMA, ensures compliance with safety and efficacy standards, expediting clinical trials and market entry.
CONCLUSION
The 1,3,4-oxadiazole nucleus has proven to be a versatile scaffold with significant therapeutic potential across a wide spectrum of biological activities, including anticancer, antibacterial, anti-inflammatory, anti-HIV, anti-tubercular, anti-diabetic, and antifungal properties. The versatility of modifications of derivatives of 1,3,4-oxadiazoles allows the suitably designed changeable characteristics of compounds, thereby enhancing their pharmacological effectiveness and expanding their applications within medicinal chemistry. The review casts attention on several synthetic approaches for developing derivatives of 1,3,4-oxadiazole under the heading of SAR-optimized therapeutic effects. The synthesis strategies discussed here illustrate how oxadiazoles can be engineered for specific biological processes and demonstrate their efficacy as antimicrobial, anticancer, and anti-inflammatory agents.
Such synthesis and functionalization methods are excellent examples that should be followed in developing 1,3,4-oxadiazole derivatives for potential therapeutic uses, thereby providing straightforward grounding for further research efforts to design those compounds to be bioavailable and specific. Further researchers of the derivatives could lead to new novel treatment options that answer emergent critical medical needs, especially infections resistant to drugs and complex diseases. Finally, 1,3,4-oxadiazole compounds represent an interesting area of research, with various pharmacological properties associated with scope in clinical therapeutics.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| I2 | = Iodine |
| EtOH | = Ethanol |
| KI | = Potassium Iodide |
| DBDMH | = 1,3-dibromo-5,5-dimethylhydantoin |
| TLC | = Thin Layer Chromatography |
| NMR | = Nuclear Magnetic Resonance |
| IR | = Infrared |
| PPA | = Polyphosphoric Acid |
| ADME | = Absorption, Distribution, Metabolism, and Excretion |
| AI | = Artificial Intelligence |
ACKNOWLEDGEMENTS
This work was partially supported by the CMU Proactive Researcher Scheme (2023) of Chiang Mai University for Sudarshan Singh.


