All published articles of this journal are available on ScienceDirect.
In-silico Characterization of Some Natural Molecules as Bioherbicides for the Control of the Parasitic Plant Cuscuta Species
Abstract
Background
Parasitic plants can cause major losses in agricultural production as an important source of biotic stress. Cuscuta is among the parasitic plant species that are widespread on all continents. The main methods used in the control of these parasitic plants are cultural measures and physical, mechanical, biological, and chemical control. This study aims to characterize plant-active molecules that have deactivation potential against the β-galactosidase enzyme, which plays a role in the attachment of Cuscuta species to the host plant and act as bioherbicides.
Methods
In this context, non-mutagenic natural plant herbicides were selected from Dr. Duke's Phytochemical and Ethnobotanical database, and then conformer screening analyses were performed using the molecular mechanics/MMFF method using Spartan software. The method was continued by performing geometry optimizations. Semi-empirical PM6 method was applied for geometry optimizations. QSAR model was created to understand the relationships between the binding energies and physicochemical properties of the studied molecules. Geometry optimizations and scoring studies of binding energies were performed in Spartan'14 and Autodock Vina 1.1.2 software, respectively.
Results
In the study, five commercial chemical herbicides used against Cuscuta spp. were selected as reference molecules and included in the studied molecule set. Later, the methods applied for candidate herbal herbicide molecules were also repeated for commercial chemical herbicide molecules and the obtained results were included in QSAR modeling. In the modeling studies, linear regression analysis was performed between the calculated physicochemical parameters of the molecule set and the binding energies. BIOVIA Discovery Studio software was used to display the results of the macromolecule-ligand docking studies.
Conclusion
According to the results obtained, among 86 plant-derived natural herbicide molecules against Cuscuta spp., Narciclasine, Deoxypodophyllotoxin, and 3-Hydroxyuridine molecules are recommended for further evaluation as natural herbicides, with confirmatory experimental steps suggested.
1. INTRODUCTION
Genus Cuscuta includes some major species and is distributed worldwide. Cuscuta species cause a considerable decrease in the yield of agricultural products. The impact of Cuscuta spp. on agricultural production is of significant consequence. Several studies have indicated that crop yield loss can range from 23% to 100%, depending on the host plant and environmental conditions [1]. The genus comprises many species that are more damaging to agriculture, including C. campestris and C. pentagona, which exhibit a global distribution [2]. It is adaptable to various climatic conditions, including tropical, subtropical, and temperate regions [3]. The Cuscuta spp. have been observed to exert particularly deleterious effects on members of the Fabaceae and Asteraceae families [4]. In addition to the detrimental parasitic impact on the plant, it serves as a vector for transmitting other pathogens to plants [5]. The management of Cuscuta spp. has been observed to result in elevated costs for farmers, accompanied by a reduction in crop yields [6]. The most prevalent species of Cuscuta in Turkey is C. campestris, which has been observed to have a wide host range encompassing over 55 plant species, including economically significant plants. Given the extensive range of hosts, C. campestris has the potential to cause significant disruption to agricultural productivity in Türkiye [7].
Members of the genus Cuscuta are rootless and leafless obligate holoparasites, and they twine around the host. They have adaptations such as adhesion to the host plant, secreting some enzymes, and inhibiting the immune response of the host [8, 9]. The photosynthesis ability of the Cuscuta species is quite limited [10]. It attaches to host plants through its specialized structure called haustorium. The haustorium attaches and penetrates the vascular tissue system of the host plant. They obtain nutrients such as water, minerals, and organic compounds. It uses the nutrients received from the host plant for growth and metabolic activities [11]. Parasitic plants interact with host plants through hormonal signaling and secondary metabolites, miRNAs, mRNAs, and small peptides [8]. Cuscuta species secrete the hormone strigolactone, which acts on the host. The strigolactone hormone promotes the formation and attachment of the haustorium [12]. The effective targeting of the Cuscuta spp. using traditional herbicide treatments is a significant challenge. This is primarily due to the robust physiological interdependence between the host and parasite, which results in collateral damage to the host plant [5, 13].
The main factor contributing to the dispersal of Cuscuta species into agricultural areas is the unintentional mixing of its seeds with the seeds of other plants with similar seed morphology. Particularly, alfalfa seeds (Medicago sativa) are confused with Cuscuta seeds and cause distribution of the plant. It is considered that Cuscuta spp. seeds were introduced into Europe with alfalfa seeds in the 1840s [14, 15]. In 1978, Nemli stated that C. campestris entered Turkey with alfalfa seeds imported from the USA in 1925 [16]. Cuscuta species spread out nearly all over the country from west to east in agricultural areas higher than the sea level, and open areas in forests, roadsides, and grasslands [17]. Furthermore, 55 different hosts of C. campestris were observed in Turkey. Some of the important agricultural hosts are shown in Table 1 [7, 16, 18].
| Plant | Common Name | Plant | Common Names |
|---|---|---|---|
| Medicago sativa L. | Alfaalfa | Vitis vinifera L. | Grapevine |
| Pimpinella anisum L. | Anise | Cucumis melo L. | Melon |
| Carum carvi L. | Caraway | Solanum tuberosum L. | Potato |
| Nicotiana tabacum L. | Tobacco | S. lycopersicum L. | Tomato |
| Cicer arietinum L. | Chickpea | Beta vulgaris L. | Sugarbeet |
| Asparagus officinalis L. | Asparagus | - | - |
Parasitic plants are a unique group of plants that pose a significant threat to agricultural production. Parasitic plants can cause significant production loss as a biotic stress factor. There are some reports about reductions in yield. In 2010, Zhao reported a 30-50% loss observed in soybean (Glycine max), in 2023, Yuan et al. reported up to 26% loss in soybean under high parasitism, in 2007, Aly reported a 50-75% loss in tomato (Solanum lycopersicum), in 2002, Marambe et al. reported a 72% loss in tomato and a 29% loss in chili (Capsicum annuum), in 20023, Moorthy et al. reported an 85.7% loss in chickpea (Cicer arietinum), and an 87% loss in lentil (Lens culinaris), and Narayana, 1989 reported 60-70% loss in alfalfa (Medicago sativa).
Currently, weed control is one of the main problems in agricultural production. The use of herbicides has become a necessity to produce adequate yields [19]. However, herbicide resistant crops are becoming an increasing problem [20]. The Herbicide Resistance Action Committee (HRAC) has categorized herbicides into 4 main groups based on 26 different Modes of Action (MoA). The main groups are herbicides affecting light activation of ROS, cellular metabolism, cell division and growth, and unknown MoA [21]. Along with increasing herbicide resistance, the development process for targeted MoA has remained relatively slow [22]. The development of new herbicides from natural resources is a promising effort to cope with the weed problem. The MoA of herbicides depends mainly on the inhibition of critical enzymes and proteins. Acetolactate synthase, Acetyl-CoA carboxylase, Serine 264 and Histidine 215 in Photosystem II (PSII), and fatty acid synthase pathways are the major MoA targets for herbicide binding and inhibition [21]. There are several potential MoA targets for the control of Cuscuta spp. such as perception proteins, hydrolytic enzymes, and cell wall organizing enzymes. β-galactosidase enzyme is involved in the cell wall organization process [23, 24]. It also takes part in the production of sticky cement at the invasion site for haustorial attachment [25]. β-galactosidase is a key enzyme that plays a critical role in the early stages of Cuscuta’s attachment to host plants. It is involved in the hydrolysis of galactosyl residues in host cell walls, which is a crucial step for facilitating parasitic invasion and establishing attachment [26]. As such, this enzyme directly impacts the ability of Cuscuta to penetrate the host tissue, making it an essential target for intervention. Furthermore, the inhibition of β-galactosidase could effectively disrupt Cuscuta’s parasitic behavior at an early stage, preventing it from successfully attaching and thereby reducing the plant's overall impact on host crops [27, 28]. The fact that this enzyme is central to the parasitism process supports its selection as a promising target for bioherbicide development.
Molecular descriptors and binding calculations of widely used Commercial herbicides (CH) were also included in this study to compare the Cndidate Bio-Herbicide (CBH) molecules. The calculated physicochemical parameters can be listed as area (Å2), volume (Å3), EHOMO (eV), ELUMO (eV), molecular weight (MW), lipophilicity (logP), polarizability (α), dipole moment (μ), hardness (η), softness (S), electronegativity (χ) and electrophilicity index (ω). According to Koopman’s theorem, the negative of the EHOMO and ELUMO corresponds to ionization potential and electron affinity, respectively. MW is a parameter directly related to the absorption of a molecule. If the MW of the candidate molecule is higher than the threshold value, the absorption decreases, indicating an inverse relationship between the two properties [29]. Furthermore, logP is one of the most important parameters taken into consideration when evaluating the drug potential of candidate molecules. Calculation of the logP parameter provides a way to interpret the hydrophobic or hydrophilic structures of the molecule [30, 31]. α parameter, which is an important parameter in understanding structure-activity relationships and chemical-biological interactions, is defined as a linear variation of the electronic charge distribution in relation to the applied electric field. The μ value, which is another physicochemical parameter taken into consideration, is a parameter that is frequently calculated in the identification of molecules and in structure-activity relationship modeling and allows us to make determinations about the electron distributions of molecules [31]. The χ concept was determined by Pauling (1960) represents the power of an atom in a molecule to attract an electron towards itself [32]. η, a measure of a molecule's potential to polarize and a chemical's resistance to changing electronic configurations, is an important quantity in the theory of chemical reactivity, as proposed by Pearson et al. (1973). On the other hand, S, which describes the potential of a molecule to accept electrons in its structure, is the measure of a chemical's propensity to change its electronic configurations according to the theory of chemical reactivity. ω value is the parameter that indicates the tendency of the molecule to accept electrons into its structure [33].
The study aims at the in-silico identification of novel CBH molecules that possess herbicidal properties by weakening parasite-host tissue compatibility through binding to the β-galactosidase enzyme, offering stronger binding affinity than current CHs. The identified natural herbicide molecules will reduce and, if possible, eliminate the damage caused by Cuscuta spp. to agricultural crops without causing any ecological contamination. The docking studies were carried out to assess the interactions between the candidate molecules and the structure of the target molecule, which is responsible for the haustorium binding mechanism of Cuscuta spp. In order to interpret the binding affinities obtained, the physicochemical parameters of the related structures were also calculated.
2. METHOD
Current commercial herbicides such as atrazine, chlorpropham, diquat, paraquat, and propyzamide were used as reference molecules in the study to evaluate the binding efficacy of the CBH. The 2D structures and SwissAdme maps of the CH are depicted in Fig. (1). Due to the unavailability of the Cuscuta sp. β-galactosidase crystal structure in the database, the Tomato β-galactosidase enzyme (PDB id: 6ik5) with a 1.82 Å resolution crystal structure was selected as the target macrostructure.Furthermore, 86 candidate ligand molecules with herbicide activity were selected from the database of Dr. Duke [34]. Additionally, 5 CHs used in the market against Cuscuta species participated in the docking study and totally examined 91 molecules. The coordinates of the active site of the β-galactosidase enzyme structure were determined as x = -19.234, y = -24.734, and z = 37.810. The SwissADME program [35] was used to evaluate the drugability potentials of the candidates. Geometry optimizations have been carried out with the semi-empirical PM6 method [36, 37] by using Spartan'14 V1.1.4 software [38]. Docking studies and visualizations were carried out with AutoDock Vina [39] and BIOVIA Discovery Studio Visualizer [40] software, respectively.
According to the obtained results via the PM6 method, the η, S, χ, and ω values were calculated by the following Eqs. (1-4) (Phillips 1961 [41]):
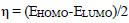 |
(1) |
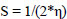 |
(2) |
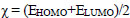 |
(3) |
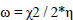 |
(4) |
3. RESULTS AND DISCUSSION
In this study, CBH molecules were determined by docking calculations. Computed physicochemical parameters of the CBH molecules, A(Å2), V(Å3), MW, EHOMO (eV), ELUMO (eV), α, μ (debye), logP, η, S, χ, ω, and BE values (kcal.mol-1), are given in Table 2.
According to the result in Table 2, the calculated physicochemical parameters of the CBH molecules included in the linear regressions were observed to vary in the range of -5.71<logp<3.01, 0.49<μ<11.95, 48.50<α<85.00, 133<Å2<560, 114.03<Å3<562.15, 110.11<MW<622.5, -4.55<η<-3.44, -0.15<S<-0.10, 4.28<χ<5.51, and -3.39<ω<2.00. In Table 2 above, the physicochemical parameter ranges for regression analysis of selected molecules with high affinity (>-7.6 kcal.mol-1) Narciclasine, Deoxypodophyllotoxin, and 3-Hydroxyuridine were performed given the physicochemical parameter ranges of the structures with high binding affinity were determined as -2.2<logp<-4.9, 3.1<μ<6.1, 57.5<α<70.8, 252<Å2<393, 225351<Å3<562.15, and 260<MW<398. Likewise, structures L-1 (Calcein) and L-2 (Asperuloside), which also show very high affinity, were not considered candidates due to SwissAdme criteria.
The results of linear regression analysis performed to determine the correlation values (R2) between the physicochemical parameter (logP, μ, α, Å2, Å3, MW) and the binding energies of CBH molecules are given in Table 3. When Table 3 is examined, the R2 values for all the critically important parameters were obtained >0.7, while the highest correlation value was calculated as R2=0.83 for MW.
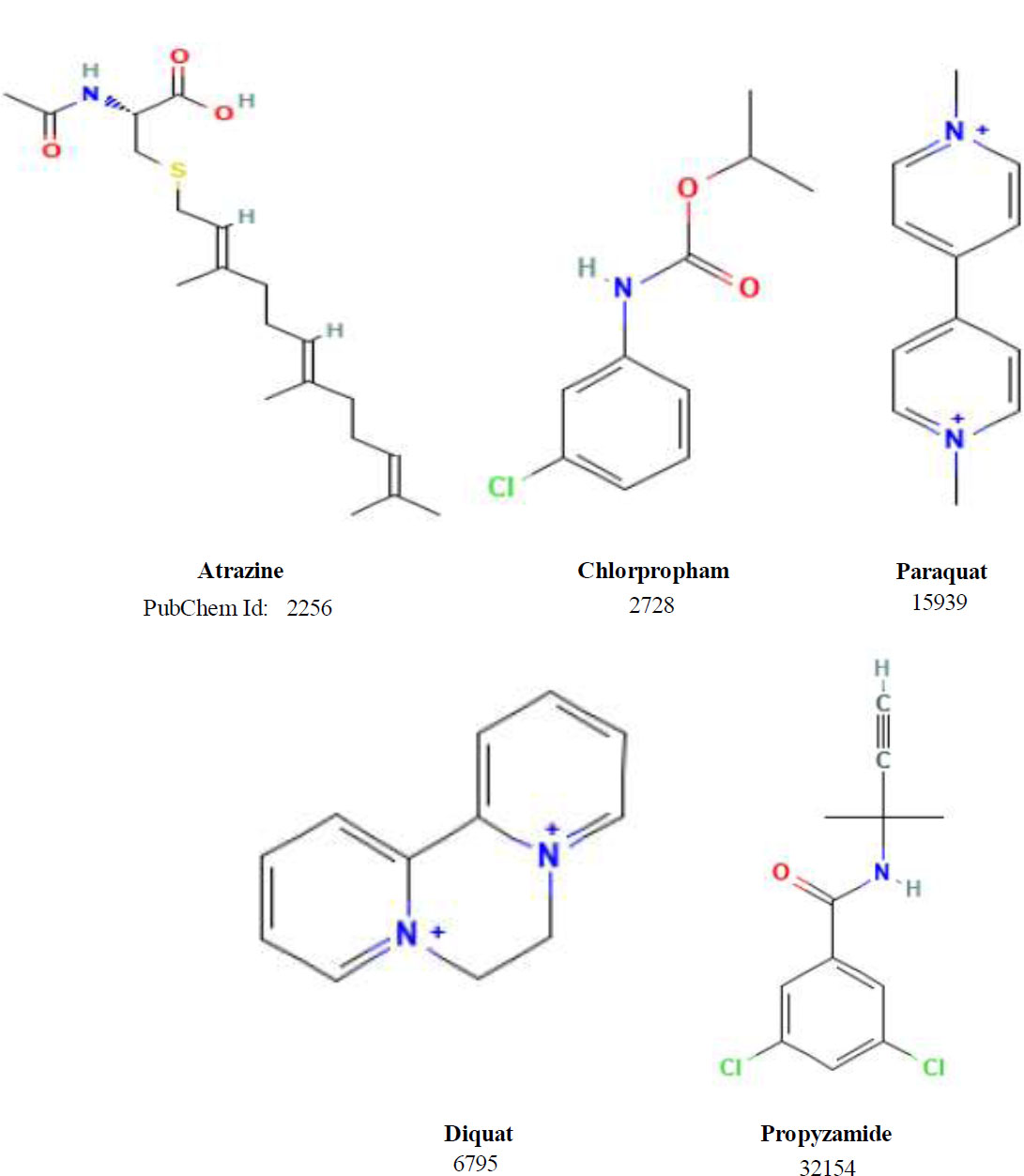
2D structure of CHs.
| CH | Ligands | Å2 | Å3 | MW | EHOMO | ELUMO | α | μ | logP | η | S | χ | ω | BE | Ki |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH-1 (Atrazine) | 249.8 | 213.0 | 215.7 | -9.4 | -0.1 | 56.5 | 3.6 | 2.8 | -4.6 | -0.11 | 4.8 | -2.4 | -5.6 | 7.8 X10-5 | |
| CH-2 (Chlorpropham) | 237.9 | 210.8 | 213.7 | -9.2 | -0.3 | 56.4 | 3.0 | 0.9 | -4.5 | -0.11 | 4.7 | -2.5 | -6.2 | 2.9 X10-5 | |
| CH-3 (Diquat) | 212.9 | 203.9 | 184.2 | -6.5 | 0.4 | 56.3 | 3.7 | -0.4 | -3.4 | -0.15 | 3.0 | -1.3 | -5.4 | 1.1 X10-4 | |
| CH-4 Paraquat | 150.9 | 128.9 | 186,3 | -9,8 | -1.3 | 49.8 | 6.4 | -0.1 | -4.3 | -0.1 | 5.5 | -3.6 | -5.4 | 1.1 X10-4 | |
| CH-5 (Propyzamide) | 269.6 | 243.5 | 256.1 | -9.9 | -0.9 | 59.0 | 3.2 | 0.9 | -4.5 | -0.11 | 5.4 | -3.2 | -5.9 | 4.7 X10-5 | |
| CBH | L-1 (Calcein) | 560.2 | 562.1 | 622.5 | -9.5 | -1.0 | 85.0 | 11.9 | -5.7 | -4.2 | -0.12 | 5.2 | -3.2 | -10.2 | 3.3X10-8 |
| L-2 (Asperuloside) | 543.3 | 446.1 | 414.4 | -8.0 | -1.1 | 75.9 | 9.2 | -2.5 | -3.4 | -0.15 | 4.5 | -3.0 | -8.1 | 1.2X10-6 | |
| L-3 (Narciclasine) | 283.5 | 268.4 | 307.4 | -9.2 | -1.2 | 61.2 | 6.1 | -4.9 | -4.0 | -0.12 | 5.2 | -3.4 | -9.9 | 5.5 X10-8 | |
| L-4 (Deoxypodophyllotoxin) | 393.1 | 387.2 | 398.4 | -8.8 | 0.2 | 70.8 | 4.1 | -3.0 | -4.5 | -0.11 | 4.3 | -2.0 | -7.7 | 2.3 X10-6 | |
| L-5 (3-Hydroxyuridine) | 252.2 | 225.1 | 260.2 | -10.0 | -1.0 | 57.5 | 3.1 | -2.2 | -4.5 | -0.11 | 5.5 | -3.4 | -7.6 | 2.7 X10-6 | |
| L-6 (Ciprofloxacin) | 333.2 | 320.6 | 331.3 | -8.6 | -0.8 | 65.5 | 9.3 | -1.6 | -3.9 | -0.13 | 4.7 | -2.9 | -7.5 | 3.2 X10-6 | |
| L-7 (Repin) | 360.1 | 351.8 | 362.4 | -10.4 | -0.5 | 67.6 | 6.7 | -0.4 | -5.0 | -0.10 | 5.5 | -3.0 | -7.1 | 6.2 X10-6 | |
| L-8 (Diboa) | 183.0 | 164.3 | 181.2 | -9.1 | -0.9 | 52.8 | 3.4 | -1.4 | -4.1 | -0.12 | 5.0 | -3.0 | -6.9 | 8.7 X10-6 | |
| L-9 (Solstitialin) | 289.0 | 278.2 | 280.3 | -10.2 | 0.1 | 61.5 | 3.8 | -0.1 | -5.1 | -0.10 | 5.0 | -2.5 | -6.6 | 1.5 X10-5 | |
| L-10 (Boa) | 261.5 | 233.7 | 207.3 | -8.4 | 0.1 | 58.3 | 5.3 | 0.0 | -4.3 | -0.12 | 4.1 | -2.0 | -6.5 | 1.7 X10-5 | |
| L-11 (Mimosine) | 213.2 | 186.2 | 198.2 | -8.8 | -0.4 | 54.5 | 5.5 | -1.5 | -4.2 | -0.12 | 4.6 | -2.5 | -6.4 | 2.0 X10-5 | |
| L-12 (Grandinol) | 267.4 | 249.6 | 252.3 | -9.6 | -0.9 | 59.6 | 2.5 | -1.4 | -4.3 | -0.12 | 5.3 | -3.2 | -6.4 | 2.0 X10-5 | |
| L-13 (Cumambrin B) | 277.1 | 270.1 | 264.3 | -9.5 | -0.4 | 61.1 | 4.9 | 0.6 | -4.6 | -0.11 | 4.9 | -2.7 | -6.3 | 2.4 X10-5 | |
| L-14 (L-Canaline) | 156.0 | 128.9 | 134.1 | -9.9 | 0.4 | 49.4 | 1.2 | -1.6 | -5.1 | -0.10 | 4.8 | -2.2 | -6.1 | 3.4 X10-5 | |
| L-15 (Benzoxazolinone) | 147.7 | 130.5 | 135.1 | -9.3 | -0.6 | 49.9 | 4.6 | -0.7 | -4.4 | -0.11 | 4.9 | -2.8 | -6.1 | 3.4 X10-5 | |
| L-16 (Indole-3-acetic acid) | 200.0 | 179.3 | 175.2 | -8.6 | -0.1 | 53.9 | 2.0 | -0.7 | -4.3 | -0.12 | 4.4 | -2.3 | -5.9 | 4.7 X10-5 | |
| L-17 (Hydroxyproline) | 150.7 | 126.1 | 131.1 | -9.5 | 0.3 | 49.3 | 1.1 | -1.2 | -4.9 | -0.10 | 4.6 | -2.1 | -5.7 | 6.6 X10-5 | |
| L-18 (Catechol) | 132.9 | 114.0 | 110.1 | -8.8 | 0.0 | 48.6 | 2.4 | -0.6 | -4.4 | -0.11 | 4.4 | -2.2 | -5.7 | 6.6 X10-5 | |
| L-19 (Methylenecyclopropylglycine) | 165.0 | 137.1 | 127.1 | -8.7 | -0.3 | 50.5 | 2.8 | -0.4 | -4.2 | -0.12 | 4.5 | -2.4 | -5.7 | 6.6 X10-5 | |
| L-20 (2-Propylquinoline) | 217.0 | 200.1 | 171.2 | -9.2 | -0.6 | 55.6 | 1.9 | 2.3 | -4.3 | -0.12 | 4.9 | -2.7 | -5.6 | 7.8 X10-5 | |
| L-21 (Cinnamic acid) | 182.1 | 160.1 | 148.2 | -9.7 | -0.9 | 52.3 | 2.8 | 1.3 | -4.4 | -0.11 | 5.3 | -3.2 | -5.6 | 7.8 X10-5 | |
| L-22 (Dehydromatricaria esther) | 235.7 | 200.9 | 172.2 | -9.6 | -1.1 | 55.7 | 4.3 | 2.0 | -4.2 | -0.12 | 5.4 | -3.4 | -5.5 | 9.3 X10-5 | |
| L-23 (Borneol) | 194.2 | 181.4 | 154.3 | -10.0 | 3.0 | 53.1 | 2.2 | 2.4 | -6.5 | -0.08 | 3.5 | -1.0 | -5.4 | 1.1 X10-4 | |
| L-24 (Pseudoisoeugenol) | 207.0 | 184.6 | 164.2 | -8.3 | 0.0 | 54.4 | 0.8 | 0.2 | -4.1 | -0.12 | 4.2 | -2.1 | -5.4 | 1.1 X10-4 | |
| L-25 (Eucalyptol) | 195.3 | 182.1 | 154.3 | -9.4 | 2.7 | 53.3 | 1.6 | 1.9 | -6.0 | -0.08 | 3.4 | -0.9 | -5.3 | 1.3 X10-4 | |
| L-26 (Beta-Pinene) | 184.1 | 170.8 | 136.2 | -9.5 | 1.5 | 52.7 | 1.0 | 3.0 | -5.5 | -0.09 | 4.0 | -1.5 | -5.3 | 1.3 X10-4 | |
| L-27 (Terpinen-4-ol) | 206.0 | 187.8 | 154.3 | -9.3 | 1.4 | 54.1 | 1.9 | 2.2 | -5.3 | -0.09 | 4.0 | -1.5 | -5.2 | 1.5 X10-4 | |
| L-28 (Limonene) | 197.4 | 176.8 | 136.2 | -9.1 | 1.2 | 53.2 | 1.0 | 3.0 | -5.2 | -0.10 | 3.9 | -1.5 | -5.2 | 1.5 X10-4 | |
| L-29 (Cinnamyl alcohol) | 179.8 | 157.9 | 134.2 | -9.3 | -0.1 | 52.0 | 1.9 | 1.2 | -4.6 | -0.11 | 4.7 | -2.4 | -5.1 | 1.8 X10-4 | |
| L-30 (Alpha-Pinene) | 184.6 | 170.8 | 136.2 | -9.0 | 1.5 | 52.8 | 0.5 | 2.9 | -5.2 | -0.10 | 3.7 | -1.3 | -5.0 | 2.2 X10-4 | |
| L-31 (Pinene) | 183.8 | 171.3 | 136.2 | -7.9 | 0.8 | 53.2 | 1.4 | 2.8 | -4.3 | -0.12 | 3.5 | -1.5 | -5.0 | 2.2 X10-4 |
| Parameter | R2 value |
|---|---|
| logP | 0.70 |
| μ | 0.70 |
| α | 0.73 |
| Å2 | 0.71 |
| Å3 | 0.72 |
| MW | 0.83 |
The interactions obtained are of critical importance for the identification of important amino acids on the active side of proteins. The interacting amino acids and bond distances are crucial in determining whether the ligands bind to the correct site in docking studies, which amino acids they interact within the active site, and in assessing the significance of mutations in potential mutation studies. Therefore, the observed interacting amino acids and the interaction types/distances between 6IK5 and CH/CBH molecules are summarized in Table 4.
| - | CH-1 | CH-2 | CH-3 | CH-4 | CH-5 | L-1 | L-2 | L-3 | L-4 | L-5 | L-6 | L-7 | L-8 | L-9 | L-10 | L-11 | L-12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y74 | - | 5.2 | - | - | - | - | - | - | - | - | - | - | 2.59 | - | - | 2.94 | - | ||
| C118 | - | 4.38 | 5.44 | - | 4.3 | - | - | - | - | - | - | 3.62 | 5.24 | - | - | 4.15 | - | ||
| E120 | 3.62 | 4.13 | - | - | 4.36 | - | - | 1.81 | - | 2.32 | - | 2.16 | 2.71 | - | 2.22 | - | - | ||
| N180 | - | - | - | - | - | - | - | - | - | 3.31 | - | - | 2.65 | - | - | - | - | ||
| E181 | 5.17 | 2.21 | - | - | - | 2.97 | - | 1.91 | - | - | - | - | - | - | - | 3.46 | 2.49 | ||
| K217 | - | - | - | - | - | - | 2.83 | - | - | - | - | - | - | - | - | - | - | ||
| C229 | - | - | - | - | - | - | 4.76 | - | - | - | - | - | - | - | - | - | - | ||
| F232 | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.08 | - | - | - | ||
| Y233 | - | - | - | - | - | 2.99 | - | - | - | - | - | - | - | 2.20 | - | - | - | ||
| E250 | - | 3.90 | - | - | - | - | - | 2.28 | - | 3.63 | - | - | 1.82 | - | - | 3.58 | - | ||
| W252 | - | - | 5.27 | - | 6.47 | 5.13 | - | - | 5.11 | - | - | - | - | - | - | 5.78 | 4.82 | ||
| W255 | 5.26 | - | 5.43 | - | 5.29 | 5.61 | - | 4.90 | 4.50 | 4.94 | - | - | - | - | 4.86 | - | - | ||
| Y256 | - | - | - | - | - | - | - | 2.71 | - | - | - | - | - | - | 4.67 | - | - | ||
| Y289 | 4.18 | - | 5.26 | - | 4.83 | - | - | - | - | 3.12 | - | - | - | - | - | 5.52 | 4.82 | ||
| I306 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.25 | - | - | ||
| Y312 | - | - | - | - | 5.18 | - | - | - | 3.63 | - | - | - | - | - | - | - | - | ||
| S379 | - | - | - | - | - | - | - | - | - | - | 2.15 | - | - | - | - | - | - | ||
| L516 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.32 | - | - | ||
| P519 | - | - | - | 5.44 | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| V548 | - | - | - | - | - | - | - | - | - | 5.15 | - | - | - | - | 4.26 | - | - | ||
| W555 | - | - | - | 4.43 | - | - | - | - | - | - | - | - | - | - | - | - | 3.99 | ||
| L560 | - | - | - | 3.42 | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| K667 | - | - | - | - | - | - | - | - | - | - | 5.16 | - | - | - | - | - | - | ||
| G671 | - | - | - | - | - | - | - | - | - | - | 3.59 | - | - | - | - | - | - | ||
| N674 | - | - | - | - | - | - | - | - | - | - | 3.00 | - | - | - | - | - | - | ||
| - | L-13 | L-14 | L-15 | L-16 | L-17 | L-18 | L-19 | L-20 | L-21 | L-22 | L-23 | L-24 | L-25 | L-26 | L-27 | L-28 | L-29 | L-30 | L-31 |
| Y27 | - | - | - | - | - | - | - | - | - | - | - | 4.07 | - | - | - | - | - | - | - |
| Y74 | - | 2.83 | - | - | - | 2.71 | - | - | 2.8 | - | - | - | - | - | - | - | - | - | - |
| C118 | - | 2.68 | 4.25 | 4.09 | - | 4.51 | - | 4.10 | - | - | - | - | - | - | 5.25 | - | - | 4.38 | - |
| A119 | - | - | - | - | 3.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| E120 | - | 2.28 | 2.13 | - | 2.46 | 2.08 | 2.52 | - | - | - | - | - | - | - | - | - | - | - | - |
| E181 | - | - | - | 2.40 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| V205 | - | - | - | - | - | - | - | - | - | - | - | 5.09 | - | - | - | - | - | - | - |
| P212 | - | - | - | - | - | - | - | - | - | - | - | 5.36 | - | - | - | - | - | - | - |
| P224 | - | - | - | - | - | - | - | - | - | - | - | 4.61 | - | - | - | - | - | - | - |
| F232 | 3.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Y233 | 2.48 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| R238 | - | - | - | - | - | - | - | - | - | - | 3.80 | - | 5.06 | 4.77 | - | - | - | - | - |
| P239 | - | - | - | - | - | - | - | - | - | - | 3.82 | - | 5.38 | 5.38 | - | - | - | - | - |
| K244 | - | - | - | - | - | - | - | - | - | - | 2.28 | - | - | - | - | - | - | - | - |
| K246 | - | - | - | - | - | - | - | - | - | - | - | - | 4.76 | 4.47 | - | - | - | - | - |
| E250 | - | - | 3.47 | - | - | 3.59 | - | 3.63 | 2.12 | - | - | - | - | - | - | - | - | - | - |
| W252 | 4.87 | - | 5.71 | 5.07 | - | - | - | 4.69 | - | - | - | - | - | - | 3.92 | - | - | 5.03 | 5.02 |
| W255 | 5.11 | - | - | 4.90 | 3.97 | - | - | 5.01 | - | - | - | - | - | - | - | - | 4.73 | 4.58 | 4.60 |
| Y256 | - | - | - | 3.02 | - | - | - | - | - | - | - | - | - | - | - | 4.52 | - | 5.25 | 5.30 |
| N282 | - | - | - | - | - | - | - | - | - | - | - | - | 3.12 | - | - | - | - | - | - |
| Y289 | - | 3.05 | 5.36 | - | - | 5.44 | - | 5.19 | - | - | - | - | - | - | - | - | 3.24 | 5.37 | 5.34 |
| L304 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.14 | - | - | - |
| I306 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.19 | - | - | - |
| Y312 | - | 2.08 | 2.70 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| L516 | - | - | - | - | - | - | - | - | - | 4.25 | - | - | - | - | - | 4.14 | - | - | - |
| P519 | - | - | - | - | - | - | - | - | - | 4.50 | - | - | - | - | - | - | - | - | - |
| V548 | - | - | - | 5.27 | - | - | - | - | 4.61 | 5.13 | - | - | - | - | 4.49 | 4.62 | - | - | - |
| W555 | - | - | - | - | - | - | - | - | - | 4.70 | - | - | - | - | - | 4.72 | - | - | - |

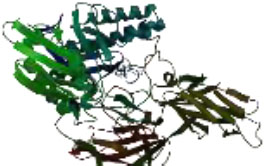
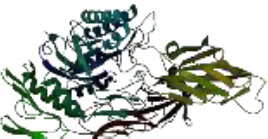
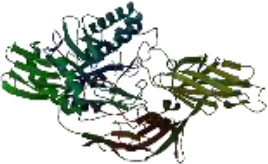
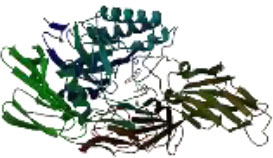
On the other hand, in a way that supports Table 4 above, it is observed that the CH/CBH agents that stand out in the docking study in the visuals given in Table 5 below are also located in the same active pocket.
Another perspective of this study is the detailed analysis of the interactions between the amino acids in the active site of the target protein structure and the candidate molecules. The interaction analysis was conducted by compiling the data obtained from the interaction maps obtained through the BIOVIA Discovery Studio software used after the docking results. According to the data, the most important amino acids in the active site of the macro-structure were determined as Tyr74, Glu120, Asn180, Glu181, Phe232, and Glu250 in types of interaction and distance. While determining these amino acids, hydrogen bonds established between molecules were taken into consideration. The formation of H bonds in the range of 1.81-5.17 Å increased the effect of these bonds on the affinity between structures. In addition, it has been calculated that π-π bonds formed with amino acids Trp252 and Trp255 containing aromatic rings are formed at a longer bond distance than H bonds. Thus, π-π bonds’ contribution to the binding energies was less. The π-alkyl bonds between an aromatic and alkyl group are mostly between the amino acids Tyr289 and Val548 of the macrostructure and the candidate molecules. The fact that these bonds are observed at a distance of 4.18-6.47 Å indicates that their contribution to affinity is less than other interactions. According to these data, the best CBHs were determined as Narciclasine, 3-Hydroxyuridine, and Deoxypodophyllotoxin for interaction with the macro-structure, as shown in Fig. (2).
To test the selectivity of the CBHs, their interactions with crucial amino acids in the active region of the macro-structure were compared to those of five commonly used CHs, which were included in the study. The amino acids with which CH molecules interact were determined as Glu120, Glu181, Trp225, Trp252, and Tyr289. In addition, it was determined that the bond distances observed in pesticide molecules were longer than the bond distances of CBH molecules with the enzyme. Additionally, when the bond types were compared, it was observed that CH molecules interacted with Glu120 amino acid with a π-anion bond at a bond distance of about 4 Å, while CBH molecules interacted with a traditional H bond at a bond distance of about 2 Å. This situation has also been observed between other important amino acids and CHs. While the binding affinity values calculated for CHs ranged from -5.4 to -6.2 kcal.mol-1, the binding affinities of the studied CBH molecules were calculated between -4.5 and -10.2 kcal.mol-1. It has been observed that CBHs interact with much more than CHs and, as a result, exhibit higher affinity. The increase in the rate of the binding affinity of the studied CBH molecules against CH molecules in the β-galactosidase inhibition is given in Table 6.
Bioherbicides, which use natural metabolites to control weeds, are a viable alternative to conventional, harmful chemical herbicides. Natural herbicides have been shown to effectively inhibit weed growth with little or no impact on the environment. The most important feature of bioherbicides is their narrow spectrum of action, i.e., their ability to control target species without harming other organisms [42]. In addition, the prevalence of herbicide-resistant weeds is increasing, and public concern about the use of chemical herbicides is growing, creating a need for the development of new natural herbicides [43]. In the last four decades, a small number of new herbicides have been introduced into agriculture. This situation requires new ways to develop efficient, biologically safe herbicides [44]. Some biotechnology companies are working on developing new pesticides by employing in-silico approaches such as artificial intelligence, machine learning, and high-throughput digital systems. The use of digital systems and an in-silico approach enables the time-efficient screening of thousands of molecules, facilitating the identification of novel herbicide candidates [45].
|
|
|
|
| Atrazine | Chlorpropham | Diquat | Paraquat |
|
|
|
|
| Propyzamide | 3-Hydroxyuridine | Deoxypodophyllotoxin | Narciclasine |
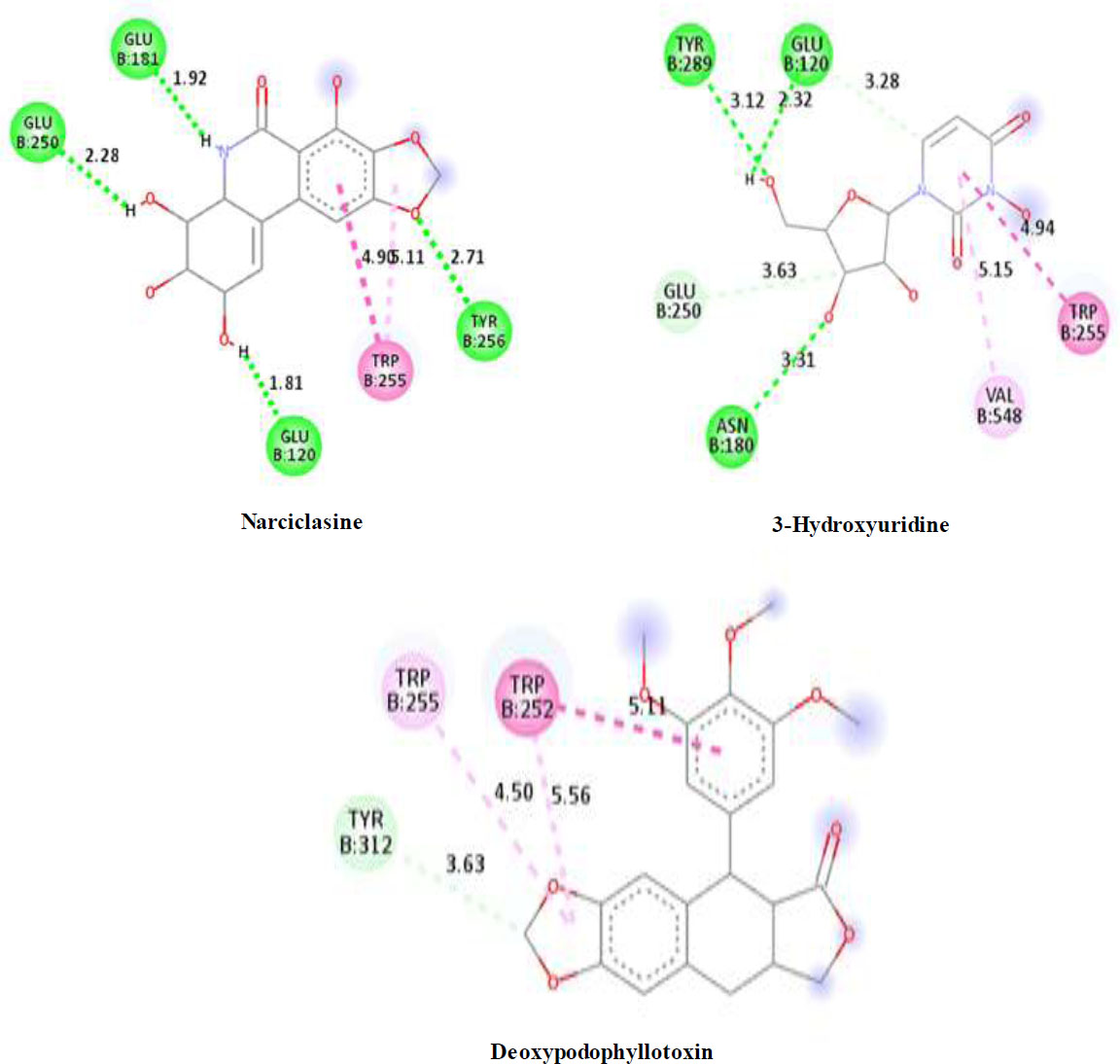
The best CBHs and interactions with the β-galactosidase enzyme.
| CBH | BEs of CBH | CH | BEs of CH | Affinity Difference | Number of CBH≥CH |
|---|---|---|---|---|---|
| Calcein | -10.2 | Chlorpropham | -6.2 | 2-10.000 | 33 |
| Narciclasine | -9.9 | Propyzamide | -5.9 | 4-20.000 | 42 |
| Deoxypodophyllotoxin | -7.7 | Atrazine | -5.6 | 8-40.000 | 55 |
| 3-Hydroxyuridine | -7.6 | Paraquat | -5.4 | 12-63.000 | 55 |
| Ilicic acid | -7.5 | Diquat | -5.4 | 12-63.000 | 55 |
| Ciprofloxacin | -7.4 | - | - | - | - |
| Podolactone B | -7.2 | - | - | - | - |
| Repin | -7.1 | - | - | - | - |
| Castanospermine | -7.0 | - | - | - | - |
| Diboa | -6.9 | - | - | - | - |
| Sandaracopimaradienediol | -6.8 | - | - | - | - |
| Balfourodinium | -6.7 | - | - | - | - |
| Solstitialin | -6.6 | - | - | - | - |
| Boa | -6.5 | - | - | - | - |
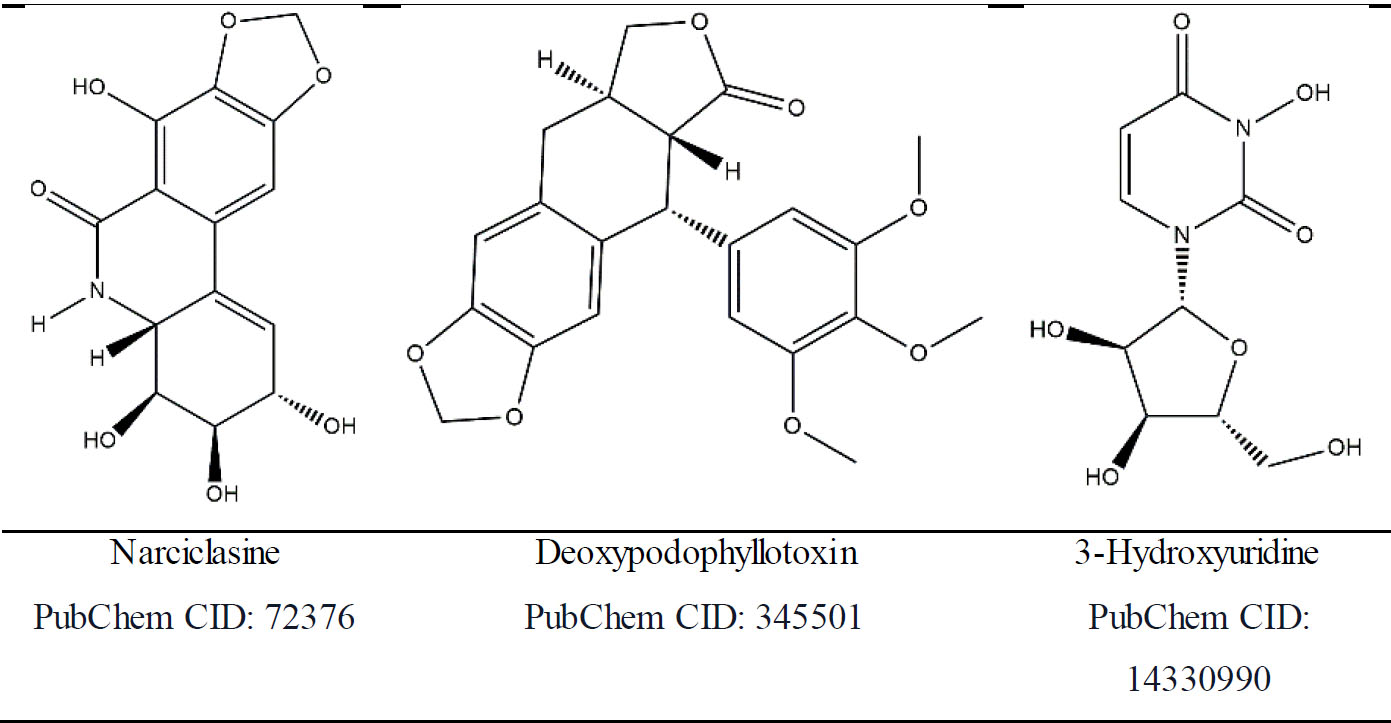
2D structures of suggested candidate molecules.
Commercial herbicides have some adverse effects on the environment and biological organisms. In this study, several commonly used herbicides, including atrazine, chlorpropham, paraquat, diquat and propyzamide, were employed for the comparison of the binding efficiency of the bio-herbicides. Atrazine is one of the most persistent herbicides, causing long-term soil and sediment pollution. With its high efficiency at a low price, it has been used all over the world [46]. Chlorpropham (CIPC) is a widely used and effective herbicide for sprout inhibition during potato storage. However, due to concerns about its toxic nature and potential public health impacts, the renewal of the CIPC has not been authorized by the European Union (EU) [47]. The European Union banned paraquat in 2007 due to its high persistence, accumulation rates, and toxicity [48]. It causes genetic, physiological, and biochemical changes, as well as oxidative stress in nearly all living cells [49]. Diquat has a structural similarity to paraquat and is also a highly persistent, non-selective herbicide. Furthermore, it has been banned in the USA and EU, and Paraquat and diquat share the same mode of action [50].
Propyzamide is a toxic and highly persistent chemical, particularly in aquatic environments. It is currently still in use. However, the EU has identified this chemical as one that may have adverse effects on various components of the environment, including groundwater, birds, mammals, and aquatic organisms [51].
The present study analyzed 86 natural plant chemicals as potential herbicide candidates and three of these were proposed as natural herbicides as a potential replacement for commercial herbicides. The chemicals proposed are narciclasine, deoxypodophyllotoxin, and 3-hydroxyuridine. Narciclasine is an alkaloid derived from the Amaryllidaceae family and has been employed in the treatment of certain tumors since ancient times. It has been demonstrated that the substance exerts inhibitory effects on the growth of other plants [52]. In a study on narciclasine conducted by Hu et al. in 2014, phytotoxic effects on lettuce (Lactuca sativa) were observed. The phytotoxic effects of narciclasine are due to its ability to arrest the cell cycle and cause DNA damage in the sample plants [53]. In another study, Qiao investigated the antifungal effects of amaryllidaceous alkaloids, including narciclasine. The authors stated that lycorine and narciclasine have high levels of antifungal activity. However, the use of narciclasine in a standalone treatment is not advised due to its phytotoxic effects [48]. Deoxypodophyllotoxin is also used for anticancer treatments for a range of cancer types due to its high cytotoxic impact on cell proliferation [55]. The other bioherbicide candidate, 3-hydroxyuridine, is derived from the Baillonella toxisperma tree and has been shown to have both phytotoxic effects and herbicidal activity. It has been reported that 3-hydroxyuridine is effective on cucumber, radish, and a variety of weeds but not on Zea mays [54]. The identification of plant CBHs via in silico methods allows for the selection of chemical substances to be carried out with greater ease and greater speed due to the use of computational power. Three chemicals were selected for further investigation as potential CBHs in replacement of prohibited and unsafe commercial herbicides to implement additional testing and application improvements.
CONCLUSION
Chemical pesticides have toxic carcinogenic and mutagenic effects. The use of natural bioherbicides is of great importance for the health and sustainability of the ecosystem [56]. Using candidate natural herbicide molecules rather than chemical herbicides is helping to cope with the deterioration of the structure of the ecosystem. The usage of in-silico methods in the investigation of biopesticide active molecules will make a great contribution in terms of both time and cost in experimental processes. Cuscuta species are an important source of biotic stress for plants with high agricultural importance. The main objective of this study is to determine the natural plant chemicals that can be used to inhibit the haustorium of Cuscuta species that feed from the host. To achieve the desired inhibition, the analysis was carried out using the docking and structure-activity analyses of CBHs. According to the results obtained from all analyses performed in the study, among all 86 candidate herbicide molecules for controlling Cuscuta sp., Narciclasine, Deoxypodophyllotoxin, and 3-Hydroxyuridine showed the highest binding affinity as -9.9 kcal.mol-1, -7.7 kcal.mol-1, and -7.6 kcal.mol-1, respectively (Fig. 3). It is suggested that these molecules be evaluated as CBH molecules due to their binding affinity, and confirmatory experimental steps should be carried out. (Fig. 3).
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: M.E.U.: Study conception and design; P.O.: Data collection; V.E.A.: Analysis and interpretation of results. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| HRAC | = Herbicide Resistance Action Committee |
| CH | = Commercial Herbicides |
| CBH | = Cndidate Bio-Herbicide |
AVAILABILITY OF DATA AND MATERIALS
All the data and supporting information is provided within the article.
ACKNOWLEDGEMENTS
Declared none.


