All published articles of this journal are available on ScienceDirect.
Assessment of Serum Angiopoietin-1 Levels During Early Stage of Missed Miscarriage
Abstract
Background
First-trimester missed miscarriage is a common complication of pregnancy that significantly impacts maternal health and healthcare services. Although fetal chromosomal anomalies constitute about half of these cases, the remaining unexplained causes mandate diagnostic investigations that explore various pathways, including angiogenesis and growth factors. One growth factor contributing to angiogenesis is Angiopoietin-1 (Ang-1).
Objective
This study aims to investigate the diagnostic role of serum levels of Ang-1 in the first trimester of missed miscarriage.
Methodology
This case-control study involved 88 pregnant women, 44 with healthy viable pregnancies at 8-14 weeks of gestation (controls), and 44 women diagnosed with missed miscarriages via ultrasound (cases) at the same gestational age. Maternal serum Ang-1 levels were recorded for comparison. The ELISA technique measured Ang-1 levels.
Results
This study revealed a statistically significant reduction in serum Ang-1 levels in missed miscarriage cases (635.75 ± 119.90 pg/mL), compared to normal pregnant women (977.13 ± 320.59 pg/mL). The cut-off point was ≤ 780.99 pg/mL, with moderate sensitivity (63.6%) and high specificity (97.9%) for distinguishing missed miscarriages from normal pregnancies. There was a strong positive correlation between Ang-1 and gestational age, suggesting a potential role of Ang-1 in normal pregnancies.
Conclusion
The study suggests a diagnostic role for Ang-1 in discriminating MM from healthy pregnancies owing to its high specificity, which reflects its role in placental development. Still, Ang-1's utility is hindered by its moderate sensitivity.
1. INTRODUCTION
When the fetus is no longer alive or growing but has not been miscarried, it is referred to as a Missed Miscarriage (MM). Eighty percent of MMs happen in the first trimester, and it affects one to five percent of pregnancies overall [1]. MM with an uncertain etiology, also known as unexplained MM or idiopathic MM, has no known cause, despite prenatal chromosomal abnormalities accounting for nearly half of the cases. This kind of MM has been associated with poor placental development because well-organized vasculogenesis and angiogenesis are required for effective placentation, and deficiencies in these processes can result in a poor pregnancy outcome [2].
The critical role of angiogenic factors in the pathophysiology of major pregnancy disorders associated with poor placentation, such as preeclampsia and fetal growth restriction, has been extensively examined and highlighted in the literature. However, their role in miscarriage remains a subject of debate, warranting further work to elucidate their potential contribution to early pregnancy losses [3].
Researchers discovered that Ang-1, a member of the angiopoietin family, has a role in the development of the fetus' circulatory system and the growth and sprouting of capillaries in the placenta and brain. During pregnancy, the placenta secretes angiopoietins, which are important in vascular endothelial development and remodeling [4]. Ang-1 is one of the growth factors promoting angiogenesis. It is secreted from the mesenchymal cells and syncytiotrophoblasts of the placenta. Ang-1 has a role in the development and branching of vessels in the fetus's trophoblastic tissue, brain, and vascular development [5].
Ultrasound has been utilized in the past and continues to be used now to determine if there is a problem with early pregnancy based on certain variables, such as gestational sac measurements and the lack of heartbeat [1]. Because miscarriages are detected by ultrasonography when a fetal heart is missing, researchers have been prompted to investigate different complements (blood biomarkers) for early detection of these problems and the prospect of prompt therapeutic intervention [5]. Researchers have demonstrated that Ang-1, a key member of the angiopoietin family, plays a crucial role in developing the fetus's circulatory system and the growth and sprouting of capillaries in the placenta and brain. Since, most MMs occur without identifiable underlying causes, it is necessary to investigate biomarkers involved in placentation and vasculature. Understanding their potential correlation with MM pathology could provide valuable insight into MM's underlying pathology, and help identify potential diagnostic and therapeutic targets [6].
The primary purpose of this study is to determine the serum level of Ang-1 in patients with MM versus Healthy Pregnancy (HP), and assess its diagnostic function in early fetal loss.
2. MATERIALS AND METHODS
A case-control study was carried out at the Yarmouk Teaching Hospital-Gynecology Clinic, from November 2023 to March 2024. The number of women who met the study criteria was 88 (44 in each group) distributed as follows:
2.1. HP Group (Healthy Pregnant, Control)
During the early stages of gestation (8-14 weeks), the diagnostic criteria for identifying a healthy and viable pregnancy within the uterus include a sac diameter of 20 mm or greater, as well as the presence of a fetal pole exhibiting a normal fetal heartbeat until the 14th week of gestation [7].
2.2. MM Group (Missed Miscarriage, Study Cases)
Diagnosed in the first trimester of pregnancy in patients who have resolved pregnancy symptoms, or may have mild vaginal bleeding verified by transvaginal sonography, and meet the diagnostic criteria for MM. Age, gravidity, gestational age, and Body Mass Index (BMI) were all matched between the study group and the control group.
2.3. Criteria of MM
The crown-rump length that is equal to or greater than 7mm with no pulsation, and the mean sac diameter that is equal to or more than 25 mm without a fetal pole on transvaginal ultrasound [5].
2.4. Exclusion Criteria
The following were the exclusion criteria: women with multiple pregnancies, induced abortion, hydatidiform mole, ectopic pregnancy, pregnancy with in vitro fertilization, medical disorders such as hypertension, diabetes, and endocrine disorders such as polycystic ovarian syndrome due to their effects on blood biomarkers, as well as inflammatory diseases; cases on chronic drugs usage, recurrent miscarriage, obese patients, and those with prior miscarriages. Cases that showed features of chromosomal abnormalities by histopathology were excluded as trophoblastic atypia and hydropic changes. The flow diagram (Fig. 1) explains the participants' selection.
The participants were clinically assessed, and those included in the study were evaluated and compared based on their Ang-1 levels. Transvaginal sonography was performed to confirm the diagnosis as per the inclusion criteria for MM, and after miscarriage, the products of conception were sent for histopathology to exclude those with abnormal changes suggestive of chromosomal abnormalities. The controls were included from those who attended the antenatal care clinic for routine visits and investigations.
All participants provided written informed consent, and the study was approved by the ETHICAL COMMITTEE: Mustansiriyah Medical College's scientific research committee, with approval no (MOG-288) on October 2023.
2.5. Sample Size Calculation
Using a confidence level of 95% (0.95), a margin of error of 5% (0.05), and a standard deviation of 0.25, the sample size required for this study was calculated using the following equation:
(1.96)2 x 0.25(0.25) / (0.05)2 = 88 participants.
Each person recruited in this study provided a random blood sample (5 mL) via vein puncture. After 15 minutes of blood clotting, blood samples were placed into disposable gel test tubes centrifuged at 3500 rpm for 10 minutes. The obtained serum was aspirated using a micropipette, and the
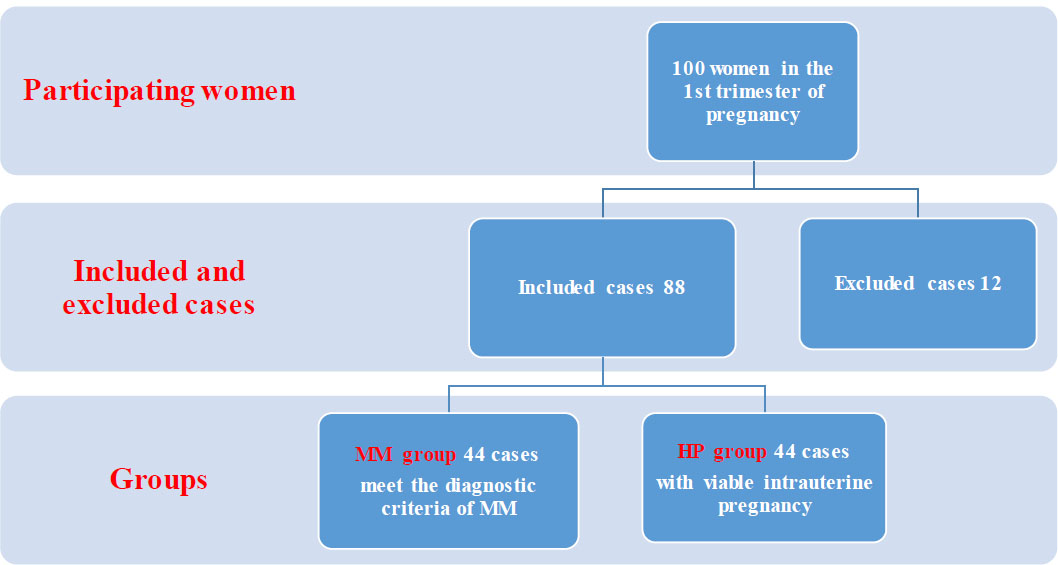
The flow diagram explains the participants' selection.
serum was transferred into sterile test tubes, labeled, and placed in a deep freeze at -80°C for biochemical analysis of Ang-1 levels, which was measured using an ELISA (enzyme-linked immunosorbent assay) kit (Bioassay Technology Laboratory, Cat. No. E1983.HU, China).
2.6. Statistical Analysis
The data was analyzed using SPSS version 25.0. The statistical significance criteria were set at a p-value < 0.05. An independent sample T-test was used to assess the differences between MM and HP regarding variables: age, gestational age, BMI, and gravida, as well as the biomarker under study, Ang-1. Linear correlation coefficients and regression models were utilized to determine the linear relationship between the biomarker Ang-1 and the other continuous variables. ROC curve analysis was used to determine the biomarkers' diagnostic performance in cases of missed miscarriage.
3. RESULTS
3.1. Demographic and Clinical Characteristics
Table 1 shows no statistically significant difference between MM and HP groups regarding age, BMI, gestational age, and gravida. However, Ang-1 levels were significantly lower in the MM group compared to the HP group (635.75 ±119.90 vs 977.13±320.59 (pg/ml), p-value = 0.0001).
Table 2 used Pearson’s correlation coefficient between Ang-1 and other data, showing a negative but insignificant association between Ang-1 and age, BMI, and gravida. In contrast, there was a substantial significant positive correlation between Ang-1 levels and gestational age.
3.2. ROC Analysis
The study's biomarkers' capacity to identify the likely incidence of MM was evaluated using the ROC curve analysis. Table 3 provides a summary of the findings.
Table 1.
| Variables | MM | HP | p-value |
|---|---|---|---|
| No. of Subjects (N) | 44 | 44 | - |
| Age (years) | 29.07 ± 6.966 | 28.27 ± 7.741 | 0.614 |
| BMI (kg/m2) | 26.98±4.024 | 27.59±3.282 | 0.443 |
| Gestational age (weeks) | 10.66±2.261 | 10.07±2.347 | 0.232 |
| Gravida | 4.77±1.764 | 4.84±2.134 | 0.87 |
| Ang-1 (pg/ml) | 635.75±119.90 | 977.13±320.59 | 0.0001 |
| Level of Ang-1 | Correlation Coefficient)r) | Age | Gestational Age | BMI | Gravida |
|---|---|---|---|---|---|
| -0.012 | 0.218 | -0.119 | -0.020 | ||
| - | p-value | 0.910 | 0.041 | 0.269 | 0.853 |
| Number | 88 | 88 | 88 | 88 |
| Angiopoietin-1 | |||||
|---|---|---|---|---|---|
| Cut off value for predicting MM | Sensitivity | Specificity | The Area under the Curve | Standard Error | p-value |
| ≤699.57 g/ml | 63.6% | 97.7% | 89.9% | 0.034 | 0.000 |
Ang-1 levels had an area under the curve (AUC) of 89.9% and a cut-off threshold of ≤699.57 pg/mL with moderate sensitivity (63.6%) and high specificity (97.9%) that were highly significant as p-value 0.000.
4. DISCUSSION
Gynecologists employ a variety of diagnostic techniques, including transvaginal ultrasound and serum human chorionic gonadotrophin testing, to screen pregnant women experiencing early vaginal bleeding or discomfort to address the huge worldwide health burden of miscarriage. Despite the convenience and practicality, their diagnostic accuracy remains restricted, even with the combination of clinical suspicion and ultrasound data [8]. This underscores the need for sensitive and reliable biomarkers to enhance early pregnancy assessment and guide clinical decisions. This research was performed to evaluate the effectiveness of Ang-1 in serum as a biomarker for MM occurring from 8 to 14 weeks of gestation.
Our study groups (HP and MM) were matched for demographic parameters, such as maternal age, gestational age, BMI, and gravidity, to reduce the influence of these variables on the study outcomes. There was no significant difference between the two groups in terms of age, BMI, gravida, and gestational age. However, a significant reduction in serum Ang-1 was observed among MM cases with a p-value of 0.00001.
The findings were consistent with the research undertaken by Destegul et al. (2020), which reported no significant variations in the correlation between maternal age and MM (p = 0.35); however, it is possible that this result was also influenced by the small sample size. The two groups did not differ significantly regarding maternal BMI [9].
Hegazy et al. (2021) demonstrated no statistically significant association between Ang-1 levels and the patient's age, gravida, and BMI, consistent with our findings [5].
A cohort of researchers determined that the BMI was statistically elevated in the missed abortion group, relative to the control group. Obese women may synthesize adipokines, proteins generated from adipose tissue involved in placental development and embryonic growth. Increased adipokine concentrations may generate a little increase in BMI, which contributes to the observed favorable impact. As a woman's BMI increases, she may experience adipokine resistance and relative insufficiency, leading to poor reproductive outcomes, such as infertility and miscarriage [10]. Zheng et al. and Rao et al. found that women with a BMI higher than 25 kg/m2 are more likely to have an abortion, which reflects the possible association with obesity [11, 12]. However, the current study controlled this confounding factor by matching BMI between the two study groups to ensure that maternal BMI does not influence observed differences.
The findings of Yang et al. (2017), who conducted a retrospective analysis of clinical data from 492 singleton pregnant women, discovered a considerably stronger connection between increased gravidity and missed abortion (p = 0.001) [13]. This finding is consistent with research conducted by Najah et al. (2020), which discovered that pregnant women with increased gravidity had a higher incidence of MM (p = 0.02) [14]. In the current work, there are no significant differences in the means of gravidity between the two groups, minimizing bias and ensuring a more accurate evaluation of the marker under study.
Our findings showed that the mean serum Ang-1 levels were significantly lower in MM women, 635.75 ± 119.90 pg/mL, whereas, in HP women, the levels were 977.13 ± 320.59 pg/mL, with a significant p-value. ROC curve analysis showed a cut-off value of ≤ 699.57 pg/mL. The specificity was high (97.7%), while the sensitivity was low (63.6%), indicating a good diagnostic performance with weaker screening profiles.
Daponate et al. found that the threshold value for Ang-1 (≤ 918.33 pg/ml) was 810.5 pg/mL in missed abortions, and 963.5 pg/ml in normal pregnant women, which is consistent with our findings [15].
The current study is similar to Rao M. et al. (2018), who found that the ideal levels of serum Ang-1 were about 780.5 pg/mL in women with MM, and 1102.5 pg/mL (1028.5 -1196.3) in normal pregnant women in the first trimester [12].
Similarly, H. M. Abozeid et al. (2021) found that the mean serum Ang-1 levels were about 700.04 pg/mL in women who had a MM, and around 926.16 pg/mL in women who had a viable intrauterine pregnancy [5].
The present study's findings on serum Ang-1 (p=0.001) align with the results of Alsamarai et al. (2016), which involved 547 women with adverse obstetric histories, and 291 women with normal pregnancy outcomes, reporting a significant association between lower levels of Ang-1 and MM (p = 0.0032) [16].
Consistent with our results, Faraj et al. (2024) measured the serum Ang-1 levels for 100 pregnant women using the ELISA method. The Ang-1 level was significantly lower in MM, compared to the control group (p<0.0001). At a cut-off point of ≤1,085 pg/mL, Ang-1 can predict MM with 88% sensitivity and 60% specificity [17]. Another study showed that the serum levels of Ang-1 are significantly decreased in pregnant women with MM compared with its level in a viable intrauterine pregnancy of comparable gestational age [18].
The explanation for these findings could be that Ang-1 plays a crucial role in the placenta's vascular growth and maturation. Therefore, low levels of Ang-1 heighten the risk of pregnancy failure by causing defective vascular formation. This impairment affects every stage of vessel formation, from sprouting to maturation, ultimately reducing the fetus's survival chances, due to insufficient exchange of nutrients and waste products. Impaired placental vascular development is associated with an imbalance in angiogenic factors, commonly observed in pathological pregnancy [12]. This imbalance is often associated with hypoxia-inducible factors that contribute to poor fetal growth and development [19], seen in complicated pregnancies and assisted reproduction strategies with higher miscarriage rates [20].
This investigation found no significant relationship between Ang-1 levels and patient’s age, BMI, and gravida, contradicting Schneuer et al.'s discovery of a positive association between Ang-1 and maternal weight [21]. Gaebler et al. discovered a significant positive correlation between Ang-1 and BMI [22]. These differences can be attributed to social and racial differences among the studied population [23, 24].
Rao et al. found that Ang-1 can detect MM without repeated measures and serves as a valuable marker [12]. This biomarker is cheap, available, simple to use, and can excel in ultrasound diagnosis, which requires further testing and follow-up to reach the diagnosis of MM at early gestation.
4.1. Strengths and Limitations of the Study
4.1.1. The Study's Strength
The study's strength lies in its focus on an important and common health problem with a diverse range of underlying etiology. It investigates the potential role of a biomarker in vascular development, angiogenesis, and vascular permeability, offering insight into its early diagnostic potential and possible future therapeutic strategies for cases with no underlying etiologies.
CONCLUSION
The current study contributes to the ongoing research for reliable markers for assessing early pregnancy, by testing the efficacy of Ang-1 as an early marker for MM between 8 and 14 weeks. The study suggests a diagnostic role for Ang-1 in discriminating MM from healthy pregnancies owing to its high specificity, which reflects its role in placental development. Nevertheless, Ang-1's utility is hindered by its moderate sensitivity. Future multicentric prospective research is warranted to validate Ang-1's predictive role, and explore possible integration into clinical protocols.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: E.A.: Study conception and design; A.A.: Data collection; B.H.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| Ang-1 | = Angiopoietin-1 |
| MM | = Missed miscarriage |
| HP | = Healthy pregnancy |
| BMI | = Body mass index |
| ELISA | = Enzyme-linked immunosorbent assay |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Committee: Mustansiriyah Medical College's scientific research committee, Iraq with approval no (MOG-288) on October 2023.
HUMAN AND ANIMAL RIGHTS
All procedures performed in the study involving human participants were in accordance with the ethical standards of institutional and/or research committee, and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [Zenodo Repository] at [https://zenodo. org/records/15379289?preview=1&token=eyJhbGciOiJIUzUxMiJ9.eyJpZCI6IjNiZGFlMzIyLWViZjctNGVmYy04Y2IwLWQ0MTgxNmE0YWQ3NCIsImRhdGEiOnt9LCJyYW5kb20iOiIwNzBkZDEyNGM0ZjRjOThlOTYxMTQ2N2VhYTkzZDlmYyJ9.tkca_QqI-wfsiy1mcJl1FUw2xvErQAeG3SRWiKbUH 1OuP6s-6ouUobC-17-F6HduTfKLfWIpnSQW8Q9fr_bLeA], reference number [5.62.145.107].
ACKNOWLEDGEMENTS
The authors are grateful to Mustansiriyah University for their assistance in finishing this study.


