All published articles of this journal are available on ScienceDirect.
Synthesis and Bronchodilator Studies of Some Novel 6-Alkyl/Aryl-1,2,4-Triazino[4,3-c]Quinazolines
Abstract
A series of alkyl- and aryl-1,2,4-triazino[4,3-c]quinazolines (5a-h and 8a-h) were synthesized and characterized. The title compounds were evaluated for their in vivo bronchodilator activity on guinea pigs. All the test compounds exhibited good protection against histamine-induced bronchospasm. The structure-activity relationships based on the results obtained for these series were studied. Incorporation of an aryl ring with halo substitution to the theophylline bioisostere increases its potency. Among the compounds tested, 5b was found to be the most potent with 88.7% protection against histamine-induced bronchospasm compared to the standard compound aminophylline (87.8%).
INTRODUCTION
Bronchial asthma is a chronic debilitating disease; in severe forms, it can even be life-threatening. It is, in general characterized by both bronchoconstriction and airway inflammation which leads to a bronchial hyperresponsiveness [1]. Despite a narrow therapeutic index, methylxanthines are the drugs of choice in asthma therapy.
Currently new tricyclic heterocyclic compounds designed on the basis of xanthine skeleton are being investigated with hopes of discovering bronchodilators with a wider margin of safety. While reviewing the recent perspectives in the design of antiasthmatic agents [2], we observed that different angularly fused heterocyclic ring systems like imidazoquinolines [3], imidazonaphthyridines [4], triazolothienopyrimidines [5], benzimidazoloquinazolines [6], imidazoquinazolines [7], benzimidazolopyridopyrimidines [8], imidazothienopyrimidines [9] and triazinoquinazolines [10] are potentially useful compounds. Previous studies with respect to xanthine derivatives suggest that an increase in the lipophilicity of the xanthine derivatives enhances bronchodilatory activity, irrespective of its side effect profile [11]. Based on these observations, a hypothetical model has been proposed (Fig. 1) with the following broad objectives: to develop xanthine-based but non-xanthine, new fused heterocycles, capable of exerting bronchodilatory effects similar to theophylline, to increase the potency and to minimize the undesirable CNS and cardiovascular effects.
Proposed hypothetical model towards the development of xanthine-based but non-xanthine heterocycles.
To achieve these objectives, we tried synthesizing a novel series of heterofused quinazolines and tested for their bronchodilatory activity. Hence we are herewith reporting the synthesis of a set of novel 6-alkyl-1,2,4-triazino[4,3-c]quinazolines and 6-aryl-1,2,4-triazino[4,3-c]quinazolines as potential bronchodilators.
MATERIAL AND METHODS
General Methods
Melting points were determined in open capillaries on a Thermonik melting point apparatus (Mumbai, India) and were uncorrected. IR spectra (KBr) (νmax; cm-1) were recorded on a Perkin Elmer spectrophotometer (577 model). 1H-NMR spectra were recorded on Bruker WM-400 spectrometer (in δ ppm) (Bruker, Flawil, Switzerland) using TMS as internal standard and mass spectra (EI-MS) on Jeol D-300 spectrometer at 70 eV. Elemental analyses were performed on Carlo-Erba 1108 elemental analyser (Heraeus, Hanau, Germany). Silica gel plates (Merck, Whitehouse station, NJ) were used to monitor the progress of the reaction, using chloroform-methanol as the mobile phase. All chemicals and reagents used in the synthesis were obtained from Sigma (Sigma-Aldrich, St. Louis MO), Lancaster (Alfa Aesar, Ward Hill, MA) or Spectrochem Pvt. Ltd. (Mumbai, India) and were used without further purification. The starting material, 2-alkyl- and 2-aryl-3,1-benzoxazin-4(3H)-ones (1a-h) were synthesized using known procedures [12, 13].
The synthesized compounds were characterized by their physical and spectral data and are given below. The in vivo bronchodilatory activity of 6-alkyl/aryl-3-oxo-[1,2,4]triazino [4,3-c] quinazolines is given in Table 1.
Bronchodilatory Activity (In Vivo) of 6-Alkyl/Aryl-3-Oxo-3,4-Dihydro-2H-[1,2,4]Triazino[4,3-c]Quinazolines (5a-h and 8a-h)
| Compounda | X | X’ | R | Mean ± SD Time of Onset of Convulsions (in Sec.) (n = 5) | % Protectionb |
|---|---|---|---|---|---|
| 5a | H | H | CH3 | 338 ± 158 | 39.77 |
| 5b | Br | H | CH3 | 1800 ± 0c | 88.70 |
| 5c | Br | Br | CH3 | 1763 ± 53 | 88.47 |
| 5d | I | H | CH3 | 1229 ±228 | 83.45 |
| 5e | H | H | C2H5 | 296 ± 61 | 31.30 |
| 5f | Br | H | C2H5 | 1688 ± 250 | 87.95 |
| 5g | Br | Br | C2H5 | 1700 ± 224 | 88.04 |
| 5h | I | H | C2H5 | 1686 ± 91 | 87.94 |
| 8a | H | H | -C6H5 | 991 ± 70 | 79.48 |
| 8b | H | H | 4-CH3-C6H4 | 626 ± 58 | 67.54 |
| 8c | H | H | 2-OCH3-C6H4 | 897 ± 52 | 77.34 |
| 8d | H | H | 3-OCH3-C6H4 | 721 ± 51 | 71.79 |
| 8f | H | H | 4-Br-C6H4 | 833 ± 100 | 75.58 |
| 8g | H | H | 4-NO2-C6H4 | 960 ± 181 | 78.81 |
| Aminophylline | 1665 ±147 | 87.79 | |||
| Control | ± 50 |
a) Dose = 50 µM/kbw
b) Percentage protection = [1-T1/T2] x 100, Where, T1 = mean time of onset of convulsions (in Sec.) of control, T2 = mean time of onset of convulsions (in Sec.) of drug treatment with respect to standard (aminophylline)
c) All five guinea pigs were stable above 1800 Sec.
Chemistry
Synthesis of 2-Alkyl/Aryl-4-Oxo-3(4H)Quinazolinacetic Acids (2a-p)
The 2-alkyl/aryl-4-oxo-3(4H)-quinazolineacetic acids (2a-p), can be synthesized by two methods (Method A & B):
Method A:
The appropriate alkyl benzoxazinone (1a-h; 0.01 mol) and glycine (0.01 mol) were ground and kept in a 50 ml beaker. To this mixture, 1-methoxy-2-(2-methoxyethoxy)ethane (2 ml) was added and the mixture was heated to 160-180 °C for 20-30 minutes. After the separation and evaporation of water from the mixture, the product was cooled down to 80 °C and ethanol (95%, 20 ml) was added through continuous trituration. The obtained product was filtered off and washed twice with cold ethanol (10 ml), once with water (50 ml), and again by ethanol (10 ml) under vacuum conditions. The product was dried and recrystallized from ethanol (95%) to yield a colorless pure product.
Method B:
To the appropriate compound 3, 1-benzoxazin-4(3H)-one (1a-h; 0.01 mol) in aqueous pyridine (50%), glycine (0.012 mol) was added and heated under reflux for 6 h. After this period, the excess pyridine was distilled off. The resulting residue was digested with hydrochloric acid (10%; 20 ml) for 2 h on a water bath. The obtained product was washed with small quantities of water and recrystallized from ethanol (95%) to yield a colorless product.
Sixteen quinazolineacetic acids (2a-p) were prepared through these methods. The Rf values were obtained from TLCs where, a chloroform/methanol mixture (3:1 v/v) was used as the mobile phase for compounds 2a-h, and chloroform/ethyl acetate (1:1 v/v) was used as the mobile phase for compounds 2i-p
2-Methyl-4-oxo-3(4H)-quinazolineacetic acid (2a)
Yield: Method A: 1.74 g (80%); Method B: 1.52 g (70%). mp: 260-263 °C (d) (Lit. m.p. 260-263 °C) [14]. TLC Rf: 0.5. IR (KBr) cm-1: 3416-3462 (br, COOH), 1680 (CO), 1585. MS m/z: 319 (M+), 326, 292, 263, 265, 253. Anal. Calcd for C13H13N2O2SBr: C, 60.60; H, 4.62; N, 12.8. Found: C, 60.33; H, 4.59; N, 12.54.
6-Bromo-2-methyl-4-oxo-3(4H)-quinazolineacetic acid (2b)
Yield: 2.30 g (78%). mp: 230-234 °C. TLC Rf: 0.43. IR (KBr) cm-1: 3415-3460 (br, COOH), 1681 (C=O), 1586. 1H-NMR (DMSO-d6) δ: 2.42 (s, 3H,CH3); 4.65 (s, 2H, COCH2); 7.61 (d, 1H, H at C-8, J = 8.1 Hz), 7.93 (dd, 1H, H at C-7, J = 8.55 Hz, 2.18 Hz); 8.51 (d, 1H, H at C-5, J = 2.38 Hz). MS m/z: 297 (M+), 295, 277, 249. Anal. Calcd for C11H9N2O3Br: C, 44.45; H, 3.05; N, 9.43. Found: C, 44.60; H, 3.22; N, 9.19.
6,8-Dibromo-2-methyl-4-oxo-3(4H)-quinazolineacetic acid (2c)
Yield: 2.82 g (75%). mp: 244-246 °C (Lit. m.p. 230 °C) [15]. TLC Rf: 0.39. IR (KBr) cm-1: 3414-3461 (br, COOH), 1682 (C=O), 1584. 1H-NMR (DMSO-d6) δ: 2.55 (s, 3H,CH3); 4.58 (s, 2H, COCH2); 8.18 (s, 1H, H at C-7); 8.30 (s, H at C-5). MS m/z: 376 (M+), 358, 330. Anal. Calcd for C11H9N2O3Br: C, 35.12; H, 2.15; N, 7.45. Found: C, 35.20; H, 2.05; N, 7.28.
6-Iodo-2-methyl-4-oxo-3(4H)-quinazolineacetic acid (2d)
Yield: 2.47 g (72%). mp: 256-258 °C. TLC Rf: 0.25. IR (KBr) cm-1: 3416-3460 (br, COOH), 1680 (C=O), 1585. 1H-NMR (DMSO-d6) δ: 2.52 (s, 3H,CH3); 4.68 (s, 2H, COCH2); 7.70 (d, 1H, H at C-8, J = 8.1 Hz), 7.98 (dd, 1H, H at C-7, J = 8.1 Hz, 2.18 Hz); 8.42 (d, 1H, H at C-5, J = 2.2 Hz). MS m/z: 344 (M+), 342, 324, 296. Anal. Calcd for C11H9N2O3I: C, 38.38; H, 2.64; N,8.14. Found: C, 38.39; H, 2.73; N, 8.30.
2-Ethyl-4-oxo-3(4H))-quinazolineacetic acid (2e)
Yield: 1.11 g (48%). mp: 225 °C (d). TLC Rf: 0.61. IR (KBr) cm-1: 3414-3459 (br, COOH), 1681 (C=O), 1585. 1H-NMR (DMSO-d6) δ: 1.38-1.41 (t, 3H, -CH2CH3, J = 8.0 Hz); 2.70-2.75 (q, 2H, CH2CH3, J = 8.0 Hz) ; 4.72 (s, 2H, COCH2); 7.70 (m, H at C-5,6,7,8). MS m/z: 232 (M+), 215, 187, 159. Anal. Calcd for C12H12N2O3: C, 62.05; H, 5.21; N,12.07. Found: C, 61.95; H, 5.03; N, 11.89.
6-Bromo-2-ethyl-4-oxo-3(4H)-quinazolineacetic acid (2f)
Yield: 1.61g (52%). mp: 246-248 °C. TLC Rf: 0.57. IR (KBr) cm-1: 3413-3460 (br, COOH), 1678 (C=O), 1584. 1H-NMR (DMSO-d6) δ: 1.41-1.45 (t, 3H, -CH2CH3, J = 7.8 Hz); 2.70-2.75 (q, 2H , CH2CH3, J = 7.9 Hz) ; 4.65 (s, 2H = 7.9 Hz) ; 4.65 (s, 2J = 7.9 Hz) ; 4.65 (s, 2H , COCH2); 7.68 (d, 1H, H at C-8, J = 8.3 Hz), 8.02 (dd, 1H, H at C-7, J = 8.3 Hz, 2.2 Hz); 8.35 (d, 1H, H at C-5, J = 2.2 Hz). MS m/z: 311 (M+), 309, 294,266. Anal. Calcd for C12H11N2O3Br: C, 46.30; H, 3.57; N, 9.01. Found: C, 46.35; H, 3.38; N, 9.23.
6,8-Dibromo-2-ethyl-4-oxo-3(4H))-quinazolineacetic acid (2g)
Yield: 2.14 g (55%). mp: 258-260 °C. TLC Rf: 0.5. IR (KBr) cm-1: 3417-3459 (br, COOH), 1680 (C=O), 1586. 1H-NMR (DMSO-d6) δ: 1.40-1.42 (t, 3H, -CH2CH3, J = 8.1 Hz); 2.70-2.75 (q, 2H , CH2CH3, J = 8.0 Hz); 4.52 (s, 2H , COCH2); 7.8 (s, 1H, H at C-7); 8.23 (s, H at C-5). MS m/z: 390 (M+), 373, 345. Anal. Calcd for C12H10N2O3Br2: C, 36.93; H, 2.59; N, 7.18. Found: C, 37.08; H, 2.65; N, 7.32.
6-Iodo-2-ethyl-4-oxo-3(4H)-quinazolineacetic acid (2h)
Yield: 1.86 g (52%). mp: 272-274 °C. TLC Rf: 0.29. IR (KBr) cm-1: 3415-3460 (br, COOH), 1679 (C=O), 1585. 1H-NMR (DMSO-d6) δ: 1.39-1.42 (t, 3H, -CH2CH3, J = 7.9 Hz); 2.67-2.72 (q, 2H , CH2CH3, J = 7.9 Hz) ; 4.62 (s, 2H, COCH2); 7.65 (d, 1H, H at C-8, J = 8.3 Hz), 8.05 (dd, 1H, H at C-7, J = 8.3 Hz, 2.2 Hz); 8.38 (d, 1H, H at C-5, J = 2.2 Hz). MS m/z: 358 (M+), 341, 294, 313, 285. Anal. Calcd for C12H12N2O3I: C, 40.23; H, 3.10; N, 7.82. Found: C, 40.33; H, 2.88; N, 7.83.
2-Phenyl-4-oxo-3(4H)-quinazolineacetic acid (2i)
Yield: Method-A: 2.1g (75%); Method-B: 1.96 g (70%); mp: 130-132 °C (d) (Lit. m.p. 120 °C) [16] TLC Rf: 0.56. IR (KBr) cm-1: 3225-3275 (br, COOH), 2930, 2610, 1686 (C=O), 1608, 1579. 1H-NMR (DMSO-d6) δ: 4.42 (s, 2H, COCH2); 7.51-7.82 (m, 9H, Ar-H). MS m/z: 280 (M+), 263, 235. Anal. Calcd for C16H12N2O3: C, 68.55; H, 4.32; N, 10.00. Found: C, 68.48; H, 4.32; N, 9.96.
2-[4-Methylphenyl]-4-oxo-3(4H)-quinazolineacetic acid (2j)
Yield: Method-A: 2.10 g (72%); Method-B: 1.08 g (37%). mp: 184-186 °C. TLC Rf: 0.60. IR (KBr) cm-1: 3230-3280 (br, COOH), 2935, 2612, 1688 (C=O), 1605, 1578. 1H-NMR (DMSO-d6) δ: 2.3 (s, 3H, PhCH3); 4.48 (s, 2H, COCH2); 7.45-7.78 (m, 8H, Ar-H). MS m/z: 294 (M+), 277, 249. Anal. Calcd for C17H14N2O3: C, 69.36; H, 4.80; N, 9.52. Found: C, 69.52; H, 4.67; N, 9.79.
2-[2-Methoxyphenyl]-4-oxo-3(4H)-quinazolineacetic acid (2k)
Yield: Method-A: 2.3 g (74%); Method-B: 1.80 g (69%). mp: 163-165 °C (Lit. m.p. 130 °C) [17]. TLC Rf: 0.49. IR (KBr) cm-1: 3228-3278 (br, COOH), 2933, 2610, 1686 (C=O), 1604, 1575, 1260, 1034. 1H-NMR (DMSO-d6) δ: 3.73 (s, 3H, OCH3); 4.38 (s, 2H, COCH2); 7.6-8.12 (m, 8H, Ar-H). MS m/z: 310 (M+), 291, 295. Anal. Calcd for C17H14N2O4: C, 65.79; H, 4.55; N, 9.03. Found: C, 66.03; H, 4.55; N, 8.84.
2-[3-Methoxyphenyl]-4-oxo-3(4H)-quinazolineacetic acid (2l)
Yield: Method-A: 2.35 g (76%); Method-B: 2.10 g (68%). mp: 179-182 °C. TLC Rf: 0.53. IR (KBr) cm-1: 3235-3280 (br, COOH), 2928, 2617, 1682 (C=O), 1600, 1578, 1262, 1032. 1H-NMR (DMSO-d6) δ: 3.53 (s, 3H, OCH3); 4.49 (s, 2H, COCH2); 7.65-8.20 (m, 8H, Ar-H). MS m/z: 310 (M+), 291, 295. Anal. Calcd for C17H14N2O4: C, 65.79; H, 4.55; N, 9.03. Found: C, 65.75; H, 4.65; N, 9.17.
2-[4-Methoxyphenyl]-4-oxo-3(4H)-quinazolineacetic acid (2m)
Yield: Method-A: 2.32 g (75%); Method-B: 2.07 g (67%). mp: 168-169 °C. TLC Rf: 0.51. IR (KBr) cm-1: 3223-3279 (br, COOH), 2935, 2614, 1675 (C=O), 1612, 1568, 1225, 1025. 1H-NMR (DMSO-d6) δ: 3.51 (s, 3H, OCH3); 4.53 (s, 2H, COCH2); 7.55-7.90 (m, 8H, Ar-H). MS m/z: 310 (M+), 291, 295. Anal. Calcd for C17H14N2O4: C, 65.79; H, 4.55; N, 9.03. Found: C, 65.83; H, 4.72; N, 9.21.
2-[4-Bromophenyl]-4-oxo-3(4H)-quinazolineacetic acid (2n)
Yield: Method-A: 2.58 g (72%); Method-B: 1.80 g (50%). mp: 177-179 °C. TLC Rf: 0.55. IR (KBr) cm-1: 3232-3276 (br, COOH), 2933, 2609, 1685 (C=O), 1605, 1582. 1H-NMR (DMSO-d6) δ: 4.75 (s, 2H, COCH2); 7.85-8.37 (m, 8H, Ar-H). MS m/z: 359 (M+), 342. Anal. Calcd for C16H11N2O3Br: C, 53.48; H, 3.09; N, 7.80. Found: C, 53.50; H, 2.99; N, 7.88.
2-[4-Nitrophenyl]-4-oxo-3(4H)-quinazolineacetic acid (2o)
Yield: Method-A: 2.60 g (80%); Method-B: 1.85 g (57%). mp: 255-257 °C. TLC Rf: 0.47. IR (KBr) cm-1: 3220-3270 (br, COOH), 2925, 2613, 1682 (C=O), 1608, 1579, 1486, 1322. 1H-NMR (DMSO-d6) δ: 5.1 (s, 2H, COCH2); 7.92-8.43 (m, 8H, Ar-H). MS m/z: 325 (M+), 308. Anal. Calcd for C16H11N3O5: C, 59.06; H, 3.41; N, 12.92. Found: C, 59.19; H, 3.45; N, 13.17.
2-[3-Chlorophenyl]-4-oxo-3(4H)-quinazolineacetic acid (2p)
Yield: Method-A: 2.26 g (72%); Method-B: 1.64 g (52%). mp: 183-185 °C. TLC Rf: 0.49. IR (KBr) cm-1: 3222-3272 (br, COOH), 2928, 2615, 1684 (C=O), 1595, 1580. 1H-NMR (DMSO-d6) δ: 4.69 (s, 2H, COCH2); 7.75-8.13 (m, 8H, Ar-H). MS m/z: 314.5 (M+). Anal. Calcd for C16H11N2O3Cl: C, 58.44; H, 3.37; N, 12.79. Found: C, 58.51; H, 3.43; N, 12.78.
Synthesis of 2-Alkyl-4-Oxo-3(4H)-Quinazolineacetic Acid Methyl Esters (3a-h)
Methanol (20 ml) was added to 2-alkyl-4-oxo-3(4H)-quinazoline acetic acid (2a-h; 0.01 mol) and cooled below 20 °C. Acetyl chloride (1.4 ml) was added to this solution drop by drop with constant shaking. The solution was heated under reflux on a water bath for one hour. Then the solution was concentrated in vacuo to half of its volume. When the hydrochloride salt began to separate, the mixture was poured onto 50 ml of crushed ice and basified with aqueous ammonia. The product was removed by two extractions with chloroform. Evaporation of the combined extracts in vacuum yielded 3a-h. This product was recrystallized with benzene/hexane (2:1 v/v) to yield 50% of colorless crystalline product. The purity of the compounds was confirmed by TLC using chloroform/methanol (9:1 v/v) as the mobile phase.
2-Methyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3a)
Yield: 1.5 g (65%). mp: 114-115 °C (Lit. m.p. 114-115 °C) [18]. TLC Rf: 0.57. IR (KBr) cm-1: 1745 (C=O), 1680 (C=O). MS m/z: 232 (M+). Anal. Calcd for C12H12N2O3: C, 62.05; H, 5.21; N,12.07. Found: C, 62.19; H, 5.17; N, 12.09.
6-Bromo-2-methyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3b)
Yield: 2.43 g (78%). mp: 131-133 °C (Lit. m.p. 131-133 °C) [19]. TLC Rf: 0.52. IR (KBr) cm-1: 1742 (C=O), 1692 (C=O). MS m/z: 311 (M+). Anal. Calcd for C12H11N2O3Br: C, 46.30; H, 3.57; N, 9.01. Found: C, 46.40; H, 3.59; N, 9.13.
6,8-Dibromo-2-methyl-4-oxo-3(4H)-quinazolineaceticacid methyl ester (3c)
Yield: 2.9 g (74%): mp: 155 °C (d). TLC Rf: 0.50. IR (KBr) cm-1: 1742 (C=O), 1692 (C=O). 1H-NMR (CDCl3) δ: 1.63 (s, 3H, COOCH3); 2.30 (s, 3H, CH3); 4.75 (s, 2H, COCH2); 8.10 (s, 1H, H at C-5), 8.29 (s, 1H, H at C-7). MS m/z: 390 (M+). Anal. Calcd for C12H10N2O3Br2: C, 36.93; H, 2.59; N, 7.18. Found: C, 36.97; H, 2.62; N, 7.22.
6-Iodo-2-methyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3d)
Yield: 1.50 g (42%). mp: 146-148 °C. TLC Rf: 0.47. IR (KBr) cm-1: 1740 (C=O), 1695 (C=O). 1H-NMR (CDCl3) δ: 1.71 (s, 3H, COOCH3); 2.54 (s, 3H, CH3); 4.68 (s, 2H, COCH2); 7.76 (d, 1H, H at C-8, J = 8.2 Hz); 8.05 (dd, 1H, H at C-7, J = 8.2 Hz, 2.1 Hz); 8.25 (d, 1H, H at C-5, J = 2.1 Hz). MS m/z: 358 (M+). Anal. Calcd for C12H12N2O3I: C, 40.23; H, 3.10; N, 7.82. Found: C, 40.00; H, 3.14; N, 7.67.
2-Ethyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3e)
Yield: 2.09 g (85%). mp: 120-122 °C. TLC Rf: 0.61. IR (KBr) cm-1: 1747 (C=O), 1678 (C=O). 1H-NMR (CDCl3) δ: 1.41-1.45 (q, 3H, -CH2CH3, J = 8.7 Hz); 1.60 (s, 3H, COOCH3); 2.70-2.75 (t, 2H, CH2CH3, J = 8.69 Hz), 4.87 (s, 2H, COCH2); 8.0-8.5 (m, 4H, Ar-H). Anal. Calcd for C13H14N2O3: C, 63.39; H, 5.73; N, 11.38. Found: C, 63.42; H, 5.65; N, 11.45.
6-Bromo-2-ethyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3f)
Yield: 1.46 g (45%). mp: 158-160 °C. TLC Rf: 0.58. IR (KBr) cm-1: 1745 (C=O), 1691 (C=O). 1H-NMR (CDCl3) δ: 1.53-1.57 (q, 3H, -CH2CH3, J = 8.5 Hz); 1.76 (s, 3H, COOCH3); 2.79-2.82 (t, 2H, CH2CH3, J = 8.5 Hz), 4.87 (s, 2H, COCH2); 7.72 (d, 1H, H at C-8, J = 8.1 Hz); 7.98 (dd, 1H, H at C-7, J = 8.1 Hz, 2.0 Hz); 8.13 (d, 1H, H at C-5, J = 2.1 Hz). MS m/z: 325 (M+). Anal. Calcd for C13H13N2O3Br: C, 48.00; H, 4.03; N, 8.62. Found: C, 48.25; H, 3.90; N, 8.76.
6,8-Dibromo-2-ethyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3g)
Yield: 2.14 g (53%). mp: 145-147 °C. TLC Rf: 0.55. IR (KBr) cm-1: 1743 (C=O), 1694 (C=O). 1H-NMR (CDCl3) δ: 1.39-1.43 (q, 3H, -CH2CH3, J = 8.65 Hz); 1.53 (s, 3H, COOCH3); 2.69-2.74 (t, 2H, CH2CH3, J = 8.65 Hz), 4.85 (s, 2H, COCH2); 8.09-8.11 (s, 1H, H at C-5), 8.29-8.31 (s, 1H, H at C-7). MS m/z: 404 (M+). Anal. Calcd for C13H12N2O3Br2: C, 38.62; H, 2.99; N, 6.93. Found: C, 38.64; H, 3.00; N, 6.89.
6-Iodo-2-ethyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3h)
Yield: 1.45 g (39%). mp: 156-158 °C. TLC Rf: 0.52. IR (KBr) cm-1: 1740 (C=O), 1695 (C=O). 1H-NMR (CDCl3) δ: 1.49-1.54 (q, 3H, -CH2CH3, J = 8.53 Hz); 1.73 (s, 3H, COOCH3); 2.74-2.78 (t, 2H, CH2CH3, J = 8.53 Hz), 4.77 (s, 2H, COCH2); 7.71 (d, 1H, H at C-8, J = 8.1 Hz); 7.93 (dd, 1H, H at C-7, J = 8.1 Hz, 2.0 Hz); 8.10 (d, 1H, H at C-5, J = 2.1 Hz). MS m/z: 372 (M+). Anal. Calcd for C13H13N2O3I: C, 41.94; H, 3.52; N, 7.53. Found: C, 42.11; H, 3.53; N, 7.61.
Synthesis of 2-Alkyl-4-Thioxo-3(4H)-Quinazolineacetic Acid Methyl Esters (4a-h)
Phosphorous pentasulphide (0.025 mol) was added to 2-alkyl-4-oxo-3(4H)-quinazolineacetic acid methyl ester (3a-h; 0.01 mol) in 1,4-dioxane (20 ml) and the reaction mixture was heated under reflux for 18-24 h. Excess solvent was distilled off under reduced pressure. The reaction mixture, on elution (column chromatography) with benzene, at first gave a resinous, obnoxious red-colored liquid. Further, the product was eluted by gradually increasing the polarity of the solvent system to benzene/chloroform/methanol (25:25:1 v/v). The product was recrystallized from chloroform/benzene mixture (1:1 v/v). The TLCs were recorded using chloroform/methanol (9:1 v/v) as a mobile phase.
2-Methyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4a)
Yield: 1.68 g (68%). mp: 158-60 °C. TLC Rf: 0.57. IR (KBr) cm-1: 3345, 1682 (C=O), 1219. 1H-NMR (DMSO-d6) δ: 2.69 (s, 3H, CH3); 3.8 (s, 3H, COOCH3); 5.2 (s, 2H, COCH2); 7.6-7.8 (m, 4H, Ar-H). IR (KBr) cm-1: 3348, 1680 (C=O), 1220. MS m/z: 348 (M+). Anal. Calcd for C12H12N2O2S: C, 58.05; H, 4.88; N, 11.29. Found: C, 57.96; H, 4.82; N, 11.32.
6-Bromo-2-methyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4b)
Yield: 2.35 g (72%). mp: 138-140 °C (Lit.m.p. 138-140 °C) [19]. TLC Rf: 0.51. IR (KBr) cm-1: 3330, 1688 (C=O), 1225. 1H-NMR (DMSO-d6) δ: 2.71 (s, 3H, CH3), 3.7 (s, 3H, COOCH3), 5.3 (s, 2H, COCH2), 7.7 (d, 1H, Ar-H, J = 8.9 Hz,), 8.1 (dd, 1H, Ar-H, J = 8.34 Hz, 2.1 Hz), 8.64 (d, Ar-H, J = 2.2). MS m/z: 327 (M+). Anal. Calcd for C12H11N2O2SBr: C, 44.04; H, 3.39; N, 8.57. Found: C, 44.25; H, 3.42; N, 8.49.
6,8-Dibromo-2-methyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4c)
Yield: 3.17 g (78%). mp: 168-170 °C. TLC Rf: 0.50. IR (KBr) cm-1: 3334, 1690 (C=O), 1228. 1H-NMR (CDCl3) δ: 1.77 (s, 3H, COOCH3); 2.48 (s, 3H, CH3); 5.5 (s, 2H, COCH2); 8.21 (s, 1H, H at C-5), 8.39 (s, 1H, H at C-7). MS m/z: 406 (M+). Anal. Calcd for C12H10N2O2SBr2: C, 44.18; H, 3.09; N, 8.59. Found: C, 44.03; H, 3.20; N, 8.52.
6-Iodo-2-methyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4d)
Yield: 2.80 g (75%). mp: 142-44 °C. TLC Rf: 0.47. IR (KBr) cm-1: 3335, 1689 (C=O), 1222. 1H-NMR (DMSO-d6) δ: 2.65 (s, 3H, CH3), 3.59 (s, 3H, COOCH3), 5.13 (s, 2H, COCH2), 7.61 (d, 1H, H at C-8, J = 8.8 Hz,), 7.9 (dd, 1H, H at C-7, J = 8.4 Hz, 2.2 Hz), 8.5 (d, H at C-5, J = 2.2). MS m/z: 374 (M+). Anal. Calcd for C12H11N2O2SI: C, 38.51; H, 2.96; N, 7.49. Found: C, 38.44; H, 3.02; N, 7.49.
2-Ethyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4e)
Yield: 1.83 g (70%). mp: 124-26 °C. TLC Rf: 0.59. IR (KBr) cm-1: 3348, 1680 (C=O), 1220. 1H-NMR (DMSO-d6) δ: 1.28-1.31 (q, 3H, -CH2CH3,J = 7.42 Hz); 2.52 (s, 3H, COOCH3); 2.67-2.71 (t, 2H, CH2CH3,J = 7.34 Hz), 4.67 (s, 2H, COCH2); 7.6-7.8 (m, 4H, Ar-H). MS m/z: 348 (M+, not recorded), 333, 299. Anal. Calcd for C13H14N2O2S: C, 59.52; H, 5.38; N, 10.69. Found: C, 59.50; H, 5.38; N, 10.78.
6-Bromo-2-ethyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4f)
Yield: 2.48 g (73%). mp: 160-162 °C. TLC Rf: 0.57. IR (KBr) cm-1: 3348, 1680 (C=O), 1220. 1H-NMR (DMSO-d6) δ: 1.34-1.38 (q, 3H, -CH2CH3,J = 7.42 Hz); 2.67-2.71 (t, 2H, CH2CH3,J = 7.34 Hz), 4.85 (s, 2H, COCH2); 7.52-7.54 (d, 1H, H at C-8, J = 7.42 Hz), 7.79-7.81 (dd, 1H, H at C-7, J = 8.3 Hz, 2.1 Hz); 8.34-8.35 (d, 1H, H at C-5, J = 2.1 Hz). MS m/z: 341 (M+, not recorded), 326, 292, 263, 265, 253. Anal. Calcd for C13H13N2O2SBr: C, 45.61; H, 3.80; N, 8.18. Found: C, 45.50; H, 3.82; N, 7.83.
6,8-Dibromo-2-ethyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4g)
Yield: 3.19 g (76%). mp: 154-156 °C. TLC Rf: 0.54. IR (KBr) cm-1: 3328, 1688 (C=O), 1225. 1H-NMR (DMSO-d6) δ: 1.44-1.47 (q, 3H, -CH2CH3,J = 7.42 Hz); 2.70-2.73 (t, 2H, CH2CH3,J = 7.40 Hz), 5.15 (s, 2H, COCH2); 7.82 (s, 1H, H at C-7); 8.4 (d, 1H, H at C-5). MS m/z: 320 (M+). Anal. Calcd for C13H12N2O2SBr2: C, 37.15; H, 2.88; N, 6.67. Found: C, 37.28; H, 2.82; N, 6.73.
6-Iodo-2-ethyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4h)
Yield: 2.87 g (74%). mp: 168-170 °C. TLC Rf: 0.50. IR (KBr) cm-1: 3337, 1685 (C=O), 1221. 1H-NMR (DMSO-d6) δ: 1.31-1.35 (q, 3H, -CH2CH3,J = 7.39 Hz); 2.61-2.65 (t, 2H, CH2CH3,J = 7.35 Hz), 4.68 (s, 2H, COCH2); 7.48-7.52 (d, 1H, H at C-8, J = 7.41 Hz), 7.75-7.79 (dd, 1H, H at C-7, J = 8.2 Hz, 2.2 Hz); 8.33-8.34 (d, 1H, H at C-5, J = 2.2 Hz). MS m/z: 388 (M+). Anal. Calcd for C13H13N2O2SI: C, 40.21; H, 3.38; N, 7.22. Found: C, 40.00; H, 3.29; N, 7.23.
Synthesis of 6-Alkyl-3-Oxo-3,4-Dihydro-2H-[1,2,4] Triazino[4,3-c]Quinazolines (5a-h)
Hydrazine hydrate (99%; 0.02 mol) was added to methyl 2-alkyl-4-thioxo-3(4H)-quinazolineacetic acid methyl ester (4a-h; 0.01 mol) in methanol (20 ml), and heated under reflux for 2-3 h. The progress of reaction was monitored by using a lead acetate paper, which turns black and also with TLC using chloroform/methanol (88:12 v/v) as a mobile phase. The mixture was cooled and the precipitated product was filtered off, washed with a little cold ethanol, dried, and recrystallized from ethanol (95%). Using the above procedure, eight 6-alkyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazolines (5a-h) were synthesized and characterized.
6-Methyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (5a)
Yield: 1.39 g (65%). mp: 298-300 °C (Lit. 298-300 °C) [18]. TLC Rf: 0.51. IR (KBr) cm-1: 3420-3460 (br, OH), 3280 (NH), 1681 (C=O), 1589 (C=N). 1H-NMR (DMSO-d6) δ: 2.42 (s, 3H, CH3); 4.72 (s, 2H, COCH2); 7.10-7.65 (m, 3H, H at C-8,9,10); 7.81-8.05 (d, 1H, H at C-11, J = 3.82 Hz); 11.00 (s, 1H, NH). MS m/z: 214 (M+, not recorded), 188, 186. Anal. Calcd for C11H10N4O: C, 61.67; H, 4.71; N, 26.15. Found: C, 61.60; H, 4.69; N, 26.12.
10-Bromo-6-methyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (5b)
Yield: 1.96 g (67%). mp: 228-230 °C. TLC Rf: 0.48. IR (KBr) cm-1: 3415-3460 (br, OH), 3275 (NH), 1664 (C=O), 1586 (C=N). 1H-NMR (DMSO-d6) δ: 2.58 (s, 3H, CH3); 5.82 (s, 2H, COCH2); 7.35-7.45 (d, 1H, H at C-8, J = 3.86 Hz), 8.04-8.06 (m, 1H, H at C-9); 8.34 (s, 1H, H at C-11). MS m/z: 293 (M+), 255, 253, 237, 223, 197, 196, 195, 182, 170, 155. Anal. Calcd for C11H9N4BrO: C, 45.07; H, 3.10; N, 19.11. Found: C, 45.22; H, 3.15; N, 19.12.
8,10-Dibromo-6-methyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline(5c)
Yield: 2.53 g (68%). mp: 184-186 °C. TLC Rf: 0.47. IR (KBr) cm-1: 3430-3480 (br, OH), 3290 (NH), 1664 (C=O), 1586 (C=N). 1H-NMR (DMSO-d6) δ: 2.60 (s, 3H, -CH3); 5.87 (s, 2H, COCH2); 8.18 (s, 1H, H at C-9); 8.30 (s, 1H, H at C-11). MS m/z: 372 (M+), 334, 332, 316, 302, 286, 285, 284. Anal. Calcd for C11H8N4Br2O: C, 35.52; H, 2.18; N, 15.06. Found: C, 35.42; H, 2.16; N, 14.92.
10-Iodo-6-methyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (5d)
Yield: 2.27 g (67%). mp: 184-186 °C. TLC Rf: 0.45. ). IR (KBr) cm-1: 3415-3460 (br, OH), 3287 (NH), 1664 (C=O), 1599 (C=N). 1H-NMR (DMSO-d6) δ: 2.56 (s, 3H, CH3); 5.81 (s, 2H ,COCH2); 7.37-7.40 (d, 1H, H at C-8, J = 7.84 Hz), 8.02-8.05 (m, 1H, H at C-9); 8.36 (s, 1H, H at C-11). MS m/z: 340 (M+, not recorded), 301, 300, 285, 258, 256, 242, 231. Anal. Calcd for C11H9N4IO: C, 38.82; H, 2.64; N, 16.47. Found: C, 38.04; H, 2.63; N, 16.02.
6-Ethyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (5e)
Yield: 1.41 g (62%). mp: 260-262 °C. TLC Rf: 0.55. IR (KBr) cm-1: 3425-3465 (br, OH), 3278 (NH), 1680 (C=O), 1585 (C=N). 1H-NMR (DMSO-d6) δ: 1.23-1.26 (q, 3H, -CH2CH3J = 8.72 Hz); 2.73-2.78 (t, 2H, CH2CH3J = 8.65 Hz), 4.72 (s, 2H, COCH2); 7.46-7.81 (m, 1H, H at C-8, 9, 10); 8.06-8.08 (d, 1H, H at C-11, J = 3.80 Hz); 9.39 (s, 1H, -NH). MS m/z: 228 (M+, not recorded), 215, 188, 187, 173, 158, 130. Anal. Calcd for C12H12N4O: C, 63.15; H, 5.26; N, 24.56. Found: C, 62.83; H, 5.69; N, 23.68.
10-Bromo-6-ethyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino [4,3-c]quinazoline (5f)
Yield: 2.03 g (66%). mp: 165-167 °C. TLC Rf: 0.51. IR (KBr) cm-1: 3420-3465 (br, OH), 3270 (NH), 1661 (C=O), 1584 (C=N). 1H-NMR (DMSO-d6) δ: 1.24-1.28 (q, 3H, -CH2CH3,J = 7.78 Hz); 2.91-2.97 (t, 2H, CH2CH3,J = 7.80 Hz), 5.76 (s, 2H, COCH2); 7.57-7.92 (m, 1H, H at C-8, 9); 8.17-8.19 (d, 1H, H at C-11, J = 3.98 Hz). MS m/z: 307 (M+), 269, 267, 251, 225, 224, 223, 210, 197, 183, 169, 155. Anal. Calcd for C12H11N4BrO: C, 46.90; H, 4.51; N, 18.24. Found: C, 47.28; H, 4.58; N, 18.49.
8,10-Dibromo-6-ethyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (5g)
Yield: 2.66 g (69%). mp: 182-184 °C. TLC Rf: 0.49. IR (KBr) cm-1: 3423-3467 (br, OH), 3272 (NH), 1659 (C=O), 1579 (C=N). 1H-NMR (DMSO-d6) δ:1.28-1.30 (t, 3H, -CH2CH3, J = 7.80 Hz); 2.95-3.00 (q, 2H, -CH2CH3, J = 7.82 Hz), 5.80 (s, 2H, COCH2); 8.18-8.19 (s, 1H, H at C-9); 8.31-8.32 (s, 1H, H at C-11); 9.40 (s, 1H, -NH). MS m/z: 386 (M+), 348, 346, 330, 316, 300, 299, 298. Anal. Calcd for C12H10N4Br2O C, 37.34; H, 2.61; N, 14.51. Found: C, 37.40; H, 2.73; N, 14.36.
10-Iodo-6-ethyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c] quinazolines (5h)
Yield: 2.37 g (67%). mp: 172-174 °C. TLC Rf: 0.47. IR (KBr) cm-1: 3413-3462 (br, OH), 3285 (NH), 1662 (C=O), 1600 (C=N). 1H-NMR (DMSO-d6) δ: 1.36-1.40 (t, 3H, -CH2CH3, J = 7.80 Hz); 3.00-3.07 (q, 2H, CH2CH3, J = 7.81 Hz), 4.85 (s, 2H, COCH2); 7.40-7.86 (m, 1H, H at C-8, 9); 8.51-8.58 (s, 1H, H at C-11, J = 3.86 Hz); 9.42 (s, 1H, -NH). MS m/z: 354 (M+, not recorded), 314, 315, 299, 272, 270, 256, 245. Anal. Calcd for C12H11N4IO: C, 40.67; H, 3.11; N, 15.85. Found: C, 40.74; H, 3.28; N, 16.10.
Synthesis of 2-Aryl-4-Oxo-3(4H)-Quinazolineacetic Acid Hydrazides (7a-h)
Thionyl chloride (5 ml) was added to 2-aryl-4-oxo-3(4H)-quinazolineacetic acid (2i-p; 0.01 mol), and heated under reflux on a water bath for 1 h. The excess thionyl chloride was removed under reduced pressure. Without further purification, the obtained acidchloride (6a-h) was added to dry pyridine (10 ml) and cooled (0 °C) using a freezing mixture. To this solution, hydrazine hydrate (99%) (0.025 mol) was added at once with vigorous stirring. The mixture was stirred for one hour and poured onto crushed ice with continuous stirring. The obtained product was filtered-off, washed with water, dried and recrystallized from ethanol (95%) to yield crystalline solid. Following the above procedure, eight acid hydrazides (7a-h) were synthesized. For TLC, chloroform/ethylacetate (1:1 v/v) was used as mobile phase.
2-Phenyl-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7a)
Yield: 2.8 g (96%). mp: 242-245 °C (d) (Lit. m.p. 245 °C) [17]. TLC Rf: 0.62. IR (KBr) cm-1: 3319 (NH), 1650 (C=O), 1604 (C=O), 1529 (C=N). 1H-NMR (CDCl3) δ:4.29 (m, 3H, NHNH2); 5.08 (s, 2H, COCH2); 7.44-8.16 (m, 9H, Ar-H). MS m/z: 294 (M+). Anal. Calcd for C16H14N4O2: C, 65.28; H, 4.80; N, 19.04. Found: C, 65.33; H, 4.72; N, 19.10.
2-(4-Methylphenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7b)
Yield: 2.52 g (82%). mp: 162-4 °C. TLC Rf: 0.65. IR (KBr) cm-1: 3325 (NH), 1640 (C=O), 1600 (C=O), 1525 (C=N). 1H-NMR (CDCl3) δ:2.3 (s, 3H, CH3); 4.35 (m, 3H, NHNH2); 4.98 (s, 2H, COCH2); 7.50-8.15 (m, 8H, Ar-H). MS m/z: 308 (M+). Anal. Calcd for C17H16N4O2: C, 66.21; H, 5.23; N, 18.18. Found: C, 66.23; H, 5.26; N, 18.21.
2-(2-Methoxyphenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7c)
Yield: 2.82 g (87%). mp: 150-152 °C. TLC Rf: 0.49. IR (KBr) cm-1: 3328 (NH), 1644 (C=O), 1608 (C=O), 1526 (C=N). 1H-NMR (CDCl3) δ:3.7 (s, 3H, OCH3); 4.35 (m, 3H, NHNH2); 4.95 (s, 2H, COCH2); 7.62-8.23 (m, 8H, Ar-H). MS m/z: 324 (M+). Anal. Calcd for C17H16N4O3: C, 66.94; H, 4.98; N, 17.28. Found: C, 67.17; H, 5.03; N, 17.32.
2-(3-Methoxyphenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7d)
Yield: 2.65 g (85%). mp: 160-162 °C. TLC Rf: 0.53. IR (KBr) cm-1: 3326 (NH), 1639 (C=O), 1607 (C=O), 1523 (C=N). 1H-NMR (CDCl3) δ:3.5 (s, 3H, OCH3); 4.42 (m, 3H, NHNH2); 5.02 (s, 2H, COCH2); 7.67-8.25 (m, 8H, Ar-H). MS m/z: 324 (M+). Anal. Calcd for C17H16N4O3: C, 66.94; H, 4.98; N, 17.28. Found: C, 66.80; H, 4.94; N, 17.16.
2-(4-Methoxyphenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7e)
Yield: 2.65 g (85%). mp: 174-176 °C. TLC Rf: 0.51. IR (KBr) cm-1: 3328 (NH), 1639 (C=O), 1597 (C=O), 1527 (C=N). 1H-NMR (CDCl3) δ:3.8 (s, 3H, OCH3); 4.61 (m, 3H, NHNH2); 5.15 (s, 2H, COCH2); 7.70-8.23 (m, 8H, Ar-H). MS m/z: 324 (M+). Anal. Calcd for C17H16N4O3: C, 66.94; H, 4.98; N, 17.28. Found: C, 66.98; H, 4.87; N, 17.19.
2-(4-Bromophenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7f)
Yield: 3.10 g (83%). mp: 168-169 °C. TLC Rf: 0.55. IR (KBr) cm-1: 3326 (NH), 1639 (C=O), 1607 (C=O), 1523 (C=N). 1H-NMR (CDCl3) δ:4.70 (m, 3H, NHNH2); 5.23 (s, 2H, COCH2); 7.68-8.19 (m, 8H, Ar-H). MS m/z: 373 (M+). Anal. Calcd for C16H13N4O2Br: C, 51.47; H, 3.51; N, 15.02. Found: C, 51.48; H, 3.63; N, 15.05.
2-(4-Nitrophenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7g)
Yield: 2.64 g (78%). mp: 239-241 °C. TLC Rf: 0.47. IR (KBr) cm-1: 3336 (NH), 1629 (C=O), 1627 (C=O), 1573 (C=N). 1H-NMR (CDCl3) δ:4.78 (m, 3H, NHNH2); 5.30 (s, 2H, COCH2); 7.72-8.31 (m, 8H, Ar-H). MS m/z: 339 (M+). Anal. Calcd for C16H13N5O4: C, 56.62; H, 3.86; N, 20.65. Found: C, 56.46; H, 3.86; N, 20.54.
2-(3-Chlorophenyl)-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7h)
Yield: 2.32 g (74%). mp: 170-172 °C. TLC Rf: 0.45. IR (KBr) cm-1: 3371 (NH), 1650 (C=O), 1604 (C=O), 1529 (C=N). 1H-NMR (CDCl3) δ:4.52 (m, 3H, NHNH2); 5.11 (s, 2H, COCH2); 7.60-8.19 (m, 8H, Ar-H). MS m/z: 329 (M+). Anal. Calcd for C16H13N4O2Cl: C, 58.44; H, 3.99; N, 17.05. Found: C, 58.47; H, 3.96; N, 17.10.
Synthesis of 6-Aryl-3-Oxo-3,4-Dihydro-2H-[1,2,4]Triazino [4,3-c]Quinazolines (8a-h)
On an oil bath, 2-aryl-4-oxo-3(4H)-quinazolineacetic acid hydrazide (7a-h; 0.01 mol) was fused at 240-250 °C for one hour. The obtained product was recrystallized from an ethanol (95%)/chloroform mixture (40:10 v/v) to yield a pure product. Eight 6-aryl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazolines (8a-h) were synthesized and characterized using this procedure. The TLCs were recorded using chloroform/ methanol (19:1 v/v) as a mobile phase.
6-Phenyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8a)
Yield: 1.39 g (65%). mp: 176-178 °C. TLC Rf: 0.72. IR (KBr) cm-1: 3455-3505 (br, OH), 3307 (NH), 1663 (C=O), 1592 (C=N). 1H-NMR (CDCl3) δ:4.99 (s, 2H, COCH2); 7.48-7.78 (m, 9H, Ar-H); 8.30 (s, 1H, -NH). 13C-NMR (CDCl3) δ: δ 77.25 (C-4), 120.14 (C-8), 126.6 (C-2' and C-6'), 127.01 (C-11a), 127.92 (C-10 and C-11), 128.19 (C-3' and C-5'), 129.22 (C-4'), 130.21 (C-9), 134.09 (C-1'), 134.44 (C-3), 147.04 (C-7a), 154.55 (C-12), 161.56 (C-6). MS m/z: 276 (M+, not recorded), 238, 237, 222, 221, 208,180. Anal. Calcd for C15H10N4O: C, 68.69; H, 3.84; N, 21.36. Found: C, 68.45; H, 4.02; N, 21.46.
6-(4-methylphenyl)-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8b)
Yield: 1.94 g (67%). mp: 148-150 °C. TLC Rf: 0.65. IR (KBr) cm-1: 3450-3503 (br, OH), 3300 (NH), 1668 (C=O), 1598 (C=N). 1H-NMR (CDCl3) δ:2.25 (s, 3H, PhCH3), 4.98 (s, 2H, COCH2); 7.52-7.84 (m, 8H, Ar-H); 8.35 (s, 1H, -NH). MS m/z: 290 (M+, not recorded), 252, 251, 236, 235, 222, 194. Anal. Calcd for C16H12N4O: C, 69.55; H, 4.38; N, 20.28. Found: C, 69.55; H, 4.22; N, 20.26.
6-(2-methoxyphenyl)-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8c)
Yield: 1.77 g (58%). mp: 132-135 °C. TLC Rf: 0.70. IR (KBr) cm-1: 3463-3515 (br, OH), 3311 (NH), 1665 (C=O), 1595 (C=N). 1H-NMR (CDCl3) δ: 3.80 (s, 3H, -OCH3), 5.04 (s, 2H, COCH2); 7.13-7.53 (m, 8H, Ar-H); 8.39 (s, 1H, -NH). MS m/z: 306 (M+, not recorded), 266, 253. Anal. Calcd for C17H14N4O2: C, 66.69; H, 4.60; N, 18.30. Found: C, 67.28; H, 4.35; N, 18.26.
6-(3-methoxyphenyl)-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8d)
Yield: 2.38 g (78%). mp: 150-152 °C. TLC Rf: 0.70. IR (KBr) cm-1: 3452-3504 (br, OH), 3310 (NH), 1646 (C=O), 1580 (C=N). 1H-NMR (CDCl3) δ: 3.86 (s, 3H, -OCH3), 5.02 (s, 2H, COCH2); 7.03-7.51 (m, 8H, Ar-H); 8.32 (s, 1H, -NH). 13C-NMR (CDCl3) δ: δ 55.45 (-OCH3), 78.5 (C-4), 114.64 (C-2'), 116.01 (C-4'), 120.16 (C-6'), 121.14 (C-8), 126.59 (11a), 127.05 (C-10 and C-11), 127.92 (C-5'), 129.37 (C-9), 134.43 (C-1'), 135.24 (C-3), 146.96 (C-7a), 154.26 (C-12), 159.38 (C-3'), 161.41 (C-6). MS m/z: 306 (M+, not recorded), 266, 253. Anal. Calcd for C17H14N4O2: C, 66.69; H, 4.60; N, 18.30. Found: C, 67.25; H, 5.05; N, 18.56.
6-(4-Methoxyphenyl)-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8e)
Yield: 2.17 g (71%). mp: 162-164 °C. TLC Rf: 0.69. IR (KBr) cm-1: 3443-3509 (br, OH), 3313 (NH), 1649 (C=O), 1582 (C=N). 1H-NMR (CDCl3) δ: 3.88 (s, 3H, -OCH3), 5.04 (s, 2H, COCH2); 7.02-7.54 (m, 8H, Ar-H); 8.38 (s, 1H, -NH). MS m/z: 306 (M+), 266, 253. Anal. Calcd for C17H14N4O2: C, 66.69; H, 4.60; N, 18.30. Found: C, 62.35; H, 4.58; N, 18.49.
6-(4-Bromophenyl)-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8f)
Yield: 2.66 g (75%). mp: 155-156 °C. TLC Rf: 0.74. IR (KBr) cm-1: 3453-3499 (br, OH), 3301 (NH), 1657 (C=O), 1585 (C=N). 1H-NMR (CDCl3) δ:4.98 (s, 2H, COCH2); 7.22-8.42 (m, 8H, Ar-H) 8.55 (s, 1H, -NH). MS m/z: 355 (M+, not recorded), 317, 302. Anal. Calcd for C15H9N4BrO: C, 52.81; H, 2.66; N, 16.42 Found: C, 52.69; H, 2.70; N, 16.61.
6-(4-Nitrophenyl)-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8g)
Yield: 2.72 g (85%). mp: 223-225 °C. TLC Rf: 0.45. IR (KBr) cm-1: 3415-3450 (br, OH), 3304 (NH), 1679 (C=O), 1550 (C=N). 1H-NMR (CDCl3) δ:4.95 (s, 2H, COCH2); 7.28-8.32 (m, 8H, Ar-H) 8.45 (s, 1H, -NH). MS m/z: 321 (M+, not recorded), 283, 268, 253, 238. Anal. Calcd for C15H9N5O3: C, 58.64; H, 2.95; N, 22.79 Found: C, 58.95; H, 3.08; N, 22.87.
6-[3-Chlorophenyl]-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (8h)
Yield: 2.52 g (81%). mp: 163-165 °C. TLC Rf: 0.43. IR (KBr) cm-1: 3425-3470 (br, OH), 3309 (NH), 1663 (C=O), 1584 (N=H). 1H-NMR (CDCl3) δ:4.94 (s, 2H, COCH2); 7.24-8.31 (m, 8H, Ar-H). 13C-NMR (CDCl3) δ: 77 (C-4), 120.23(C-8), 126.66 (C-6'), 127.32 (C-2'), 127.61 (C-11a), 127.96 (C-10 and C-11), 129.36 (C-5'), 129.59 (C-4'), 130.27 (C-9), 134.16 (C-3'), 134.61 (C-1'), 135.69 (C-3), 146.88 (C-7a), 153.34 (C-12), 161.62 (C-6). MS m/z: 311 (M+, not recorded), 273, 258. Anal. Calcd for C16H11N4OCl: C, 61.83; H, 3.54; N, 18.04. Found: C, 61.77; H, 3.72; N, 18.27.
Pharmacology
General Methods
Heartly guinea pigs of either sex (7-8 weeks old, 250-300g) were obtained from National Institute of Nutrition (Hyderabad, India), and housed in wire-mesh cages in a restricted access room, under constant conditions (23 ± 2°C 12 h light) for 3-4 days before the experiments. The animals were fed standard lab pallets (Hindustan Lever Ltd., Mumbai) and purified water ad libitum. Prior to the studies, the animals were fasted for 24 h (i.e., they were deprived of food but maintained on purified water). All the animal experiments were carried out according to internationally valid guidelines, with approval from the ‘Institutional Animal Ethics Committee’ of the university.
Bronchodilator Activity: Protection Against Histamine-Induced Bronchospasm on Conscious Guinea Pigs (In Vivo Model)
The bronchodilator activity was carried out for all the title compounds by in vivo method. For in vivo activity, a histamine chamber method [20] was used. Aminophylline was used as standard bronchodilator.
A modification of the technique of van Arman et al, was used. A group of five albino guinea pigs of either sex (250-300 g) were fasted for 24 h. The test compounds were administered intraperitoneally at a dose of 50 μM/kilo body weight (kbw) and challenged with a histamine aerosol (0.2% aqueous solution of histamine acid phosphate in a Vaponephrin Pocket Nebulizer, Inco Instruments, Ambala, India) sprayed into a closed transparent cage. The respiratory status, reflecting the increasing degree of bronchoconstriction, was recorded. The observations were made for 30 minutes. The time of the onset of convulsions was recorded. Animals remaining stable (appearing normal, without increased respiratory rate and convulsions) for more than 6 minutes were considered protected against histamine-induced bronchospasm. An intraperitoneal injection of chlorpheneramine maleate at a dose of 25 mg/kbw was given for the recovery of the test animals. Aminophylline (50 μM/kbw) was used as a standard bronchodilator for comparison. The IC50 values have been expressed in terms of percentage protection (Table 1).
RESULTS AND DISCUSSIONS
Chemistry
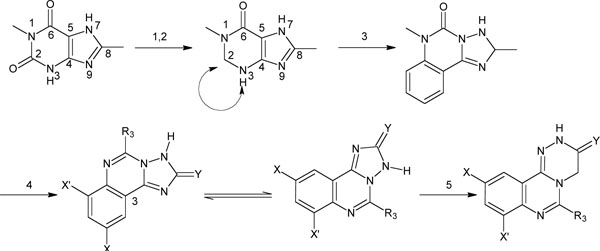
A literature survey revealed different procedures for the construction of 1,2,4-triazinoquinazolines, using thermal cyclizations [21], cyclocondensation [22], dehydro cyclization [23] and some different synthetic methodologies [24,25]. The synthesis of 6-alkyl-1,2,4-triazino[4,3-c]quinazolines 5a-h has been effected as depicted in (Fig. 2) and that of 6-aryl-1,2,4-triazino[4,3-c]quinazolines 8a-h in (Fig. 3). 1,2,4-triazino[4,3-c]quinazolines were synthesized by condensing benzoxazinones 1a-h with glycine in presence of 1-methoxy-2-(2-methoxyethoxy)ethane or aqueous pyridine to give substituted quinazolineacetic acids 2a-h. The IR spectra of Compound 2 showed characterisitic broad carboxylic acid peak at around 3410 cm-1. The incorporation of glycine was further confirmed by the NMR spectra with a peak at 4.6, corresponding to CH2-C=O group and by mass spectra.
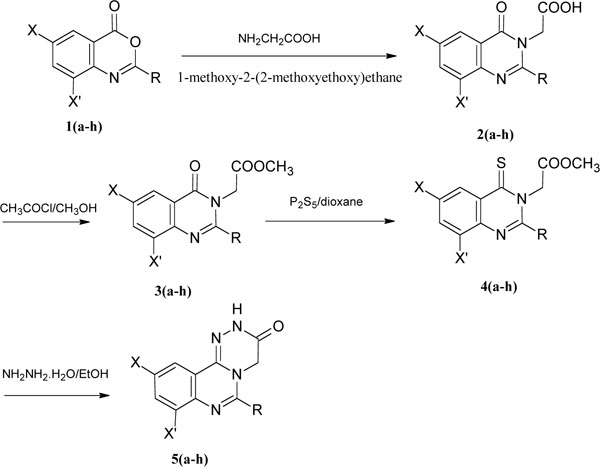
Synthesis of 6-alkyl-1,2,4-triazino[4,3-c]quinazolines 5(a-h).
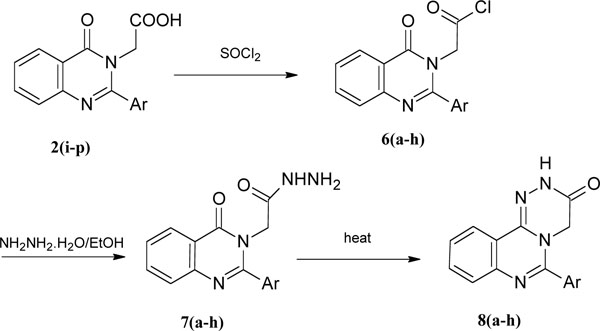
Synthesis of 6-aryl-1,2,4-triazino[4,3-c]quinazolines (8a-h).
Upon confirmation of Compound 2 spectral data, they were converted to their corresponding esters 3a-h. Compound 3 were confirmed by the disappearance of the broad carboxylic acid peak in the IR spectra and by appearance of a methyl group as a singlet at δ 1.7 ppm in 1H NMR spetra. The mass spectra showed the molecular ion peak. Compound 3, by heating under reflux in phosphorous pentasulphide were converted to 4a-h their thioxo derivative, confirmed by the spectral data. Upon cyclization with hydrazine hydrate, compound 4 yielded compound 5a-h ranging from 65 to 69%. In the IR spectrum, the disappearance of C=S peak in compound 4a-h showed the completion of the reaction to give compound 5a-h.
Alternatively 2 were converted to their corresponding acid chlorides 6 in presence of thionyl chloride. Acid chloride 6, without further purificaition, on treatment with hydrazine hydrate gave the hydrazides 7a-h. The hydrazides showed a characteristic multiplet at δ 4.7 ppm in 1H NMR spectra. Compound 7, in turn cyclized in ethanol gave 6-aryl-1,2,4-triazino[4,3-c]quinazolines 8a-h. The yields were 58-85%. Thus the present route, being advantageous in simple reaction conditions and easy work-up procedures, has resulted in improved yields.
The title compounds 5 and 8 showed a peak at around 3480 cm-1 as a broad weak signal corresponding to the tautomeric carbonyl amido group in their IR spectra. At around 3200-3300 cm-1 a peak appeard for secondary amino group and at 1650 cm-1 a peak appeard for amide carbonyl group. This further revealed the formation of the expected product. The 1H NMR spectrum showed a singlet at δ 4.86 ppm corresponding to methylene protons at C-4. A doublet of doublet appeared at δ 7.78-7.82 ppm corresponding to the proton at C-9. Also, two doublets appeared at δ 7.53-7.56 ppm and δ 8.36 ppm, corresponding to two protons at C-8 and C-11 respectively for unsubstituted triazinoquinazolines. The NH proton could be recorded below δ 9 ppm. In CMR spectrum, characteristic peaks were observed to due ketoenol tautomerism of –COCH2- group. The signal of the tertiary carbon of HO-C(NH)=CH- appeared at 147 δ ppm and of secondary carbon appeared at 77 δ ppm.
Though the mass spectrum [EI-MS] did not show a molcular ion peak (M+) the fragmentation pattern is characteristic to its structure. The loss of carbon suboxide (C2O) was found to be a common path in triazino[4,3-c]quinazolines. The fragmentation pattern of 10-bromo-6-methyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-c]quinazoline (5a) is explained. The molecular ion of compound 5a was recorded at m/z 307 with a relative abundance of 1%. On losing carbon suboxide (C2O), molecular ion produced a base peak at m/z 267. By losing an amino group, the base peak yielded m/z 251 with a relative abundance of 37%. Further, on losing a cyano group and two protons sequantially produced ions at m/z 225 (14%), m/z 224 (20%) and m/z 223 (15%). The ion m/z 223 on lose of cyanide radical gave a peak at m/z 197 with a relative abundance of 68%. Further m/z 197, on subsequential losses of two methylenes and a nitrogen radical produced ions m/z 183 (13%), m/z 169 (13%) and bromo benzine radical (m/z 155; 15%) respectively.
Pharmacology
Protection Against Histamine-Induced Bronchospasm on Conscious Guinea Pigs (In Vivo Model)
Sixteen compounds of triazino-fused quinazolines containing both alkyl and aryl substitutions with halo substitutions on the quinazoline ring were evaluated for bronchodilator activity at the dose level of 50 μM/kbw based on the reported methods [20]. Most of the compounds were found to exhibit bronchodilator activity in guinea pig (in vivo) models. Percentage protection data showed that all the test compounds of series have shown protection in the range of 31-89%.
To find out the statistical significance between data, a one-way ANOVA (Dunnett’s test) was performed. The compounds 8e and 8h were found to be inactive. Structure activity relationship (SAR) studies indicated that the alkyl and aryl substitutions at 6th position of triazino[4,3-c]quinazolines and substituents at 8th and 10th position of triazinoquinazoline ring found to exert varied biological activity. Among the 6-aryl-triazino[4,3-c]quinazolines, compounds 8a, c and g (79.48, 77.34 and 78.81% protection respectively) were found to be comparably potent to the standard (aminophylline). Among the 6-alkyl-triazino[4,3-c]quinazolines, 6-ethyl derivatives were less potent than their corresponding methyl analogues. 8, 10-halo substitution increased their activity levels compared to unsubstituted compounds, and found to be equipotent or more potent than aminophylline.
All the test compounds have exhibited bronchodilatory activity in in vivo evaluation and most of the compounds were found to be equipotent when compared with the reference compound. The design of the title compounds, as indicated, was done on the basis of xanthine skeleton. Although lacking a xanthine nucleus in their structures, these compounds exhibited bronchodilatory effects similar to xanthine derivatives. The results of SAR studies show identical features in both the series of the title compounds. The presence of electron withdrawing groups enhanced the potency, in general. Hence, bromo-substituted compounds were the most potent in alkyl series and a nitro substitution on phenyl ring seems to increase the activity. This reveals that the incorporation of an aryl ring with halo substitution, to the theophylline bioisostere increases its potency.
CONCLUSION
In conclusion, we have designed and synthesized a set of novel 6-alkyl/aryl triazino[4,3-c] quinazolines as possible bronchodilators. Although they lack a xanthine nucleus in their structure, the quinazolines exhibited bronchodilatory effects similar to xanthine derivatives. All the test compounds exhibited bronchodilatory activity in in vivo evaluation. Further studies are in progress to determine the exact mechanism of these molecules, and to explore other possible isosteres.
ACKNOWLEDGEMENT
We wish to thank the Principal, University College of Pharmaceutical Sciences, Kakatiya University, Warangal, for providing facilities to carry out this work. One of the authors (RSK) is grateful to UGC, New Delhi, for receiving a Junior Research Fellowship.


