All published articles of this journal are available on ScienceDirect.
The Therapeutic Potential of D-Amino Acid Oxidase (DAAO) Inhibitors
Abstract
D-amino acid oxidase (DAAO) is a flavoenzyme that degrades D-amino acids through the process of oxidative deamination. DAAO regulation of D-amino acid levels has been associated with several physiological processes ranging from hormone secretion to synaptic transmission and cognition. Recent genetic studies have identified a mutation on chromosome 13 in schizophrenia patients that encodes two gene products (G30 and G72) that are associated with DAAO. Furthermore, DAAO expression and enzyme activity has been reported to be increased in post mortem brain tissue samples from patients with schizophrenia compared to healthy controls. D-serine, a D-amino acid that is regulated by DAAO, is a potent, endogenous co-agonist of the N-methyl-D-aspartic acid (NMDA) receptor. Because NMDA receptor dysfunction is thought to be involved in the positive (psychotic), negative and cognitive symptoms in schizophrenia, there has been much interest in developing potent and selective DAAO inhibitors for the treatment of this disease. Several research reports have been published that describe the synthesis and biological effects of novel, selective, small molecule inhibitors of DAAO. Many of these compounds have been shown, when given systemically, to increase D-serine concentrations in the blood and brain. However, the efficacy of these compounds in behavioral assays that measure antipsychotic potential and pro-cognitive effects in laboratory animals has been inconsistent. This article highlights and reviews research advances for DAAO inhibitors published in peer reviewed journals.
D-AMINO ACID OXIDASE
D-amino acid oxidase (DAAO) is a flavoprotein that catalyzes the oxidative deamination of D-amino acids. DAAO was first identified by Krebs in 1935 [1] and since this initial characterization DAAO has been studied in a variety species ranging from microorganisms to mammals. The DAAO gene has been mapped to chromosome 12q23-24.1 in human and to 5E3-F in the mouse [2]. Several regions of the DAAO protein are highly conserved across species including amino acid residues that make up the catalytic site of the active center. However, there is no significant amino acid homology within the substrate binding region of DAAO which may reflect the broad substrate specificity of this enzyme [3]. Interestingly, DAAO can maintain catalytic activity as a monomer and a homodimer in different cell types [4, 5].
High levels of DAAO expression and enzyme activity are found in the mammalian liver, kidney, and brain although the expression pattern can vary between species. For example, humans express DAAO in both the liver and kidney, whereas mice express DAAO in the kidney but not the liver [3]. The physiological role of DAAO in the kidney and liver is detoxification of accumulated D-amino acids [6]. The cellular localization of this enzyme in peroxisomes allows for efficient removal of the cytotoxic by-product hydrogen peroxide that is produced during the oxidative deamination of D-amino acids [7]. The physiological role of DAAO in the brain is less clear. DAAO expression and activity has been reported to be most extensive in the cerebellum followed by the dorsolateral prefrontal córtexhippocampus and substantia nigra [8, 9]. Verrall et al. 2007 reported robust mRNA expression and immunoreactivity for DAAO in Bergmann glia and in the molecular and granule cell layers of the human cerebellum. In contrast to the cerebellum, DAAO mRNA and protein were localized to neurons in the prefrontal cortex, hippocampus and substantia nigra [10].
POTENTIAL THERAPEUTIC APPLICATIONS OF DAAO INHIBITORS
DAAO and the D-amino acids that this enzyme regulates have been implicated in a wide array of physiological processes. In particular D-arginine affects pathways that regulate arterial pressure [11], D-aspartate is an important regulator of hormone release [12, 13], and elevated D-alanine content has been found in the gray matter of Alzheimer's patients [14]. Recently, research has focused on understanding the role of DAAO and D-amino acids in relation to psychiatric disorders such as schizophrenia. In particular, D-serine and glycine play an important role in neuronal signaling by functioning as co-agonists of the NMDA receptor. The NMDA receptor functions as a molecular coincidence detector and requires the presence of both agonist (glutamate) and co-agonist (D-serine, glycine, and/or D-alanine) for the ligand-gated ion channel to open [15-17]. Importantly, D-serine has been reported to be the predominant NMDA co-agonist in the forebrain and there is accumulating evidence that D-serine regulates cortical and hippocampal NMDA receptor activity [17-19].
Regulation of NMDA receptor co-agonists through the pharmacological manipulation of DAAO and glycine transporters (GlyT1) have been investigated as putative novel therapeutics to treat schizophrenia. Currently, typical and second generation atypical antipsychotics are the frontline of treatment for schizophrenia. These therapeutics are moderately effective in treating the positive symptoms of schizophrenia, however, they fall short of addressing the cognitive deficits and negative symptoms associate with this disease [20]. Therapeutics that modulate D-serine and other NMDA receptor co-agonists may better address the multiple symptomatic domains of schizophrenia. The NMDA receptor is thought to play a central role in the pathophysiology of schizophrenia and NMDA receptor dysfunction may underlie the behavioral and neurobiological deficits observed in this disease. Accordingly, decreasing NMDA receptor function by administering NMDA receptor antagonists such as ketamine and phencyclidine (PCP) produced psychotomimetic symptoms, negative symptoms and cognitive deficits in animals and normal human subjects [21-23]. NMDA receptor antagonists also reinstated schizophrenia-like symptoms in remitted patients and exacerbated psychosis in patients free of antipsychotic medication [24]. D-serine, as well as other endogenous co-agonists of the NMDA receptor, are decreased in both the serum and cerebrospinal fluid (CSF) of patients with schizophrenia [25-28]. Furthermore, increasing NMDA receptor function by co-administration of glycine, D-serine, or D-alanine with atypical antipsychotics improved positive, negative, and cognitive symptoms, in schizophrenia patients when compared to antipsychotic treatment alone [29-32].
Gene association studies have provided another link between DAAO and schizophrenia. DNA from 213 schizophrenic patients and 241 normal individuals from Canada were genotyped and two overlapping gene products, G72 and G30, were identified in a segment from chromosome 13q34 that has been genetically linked to schizophrenia [33]. The G72 gene product was found to increase activity of DAAO and was subsequently termed D-amino acid oxidase activator (DAOA) [33]. This finding suggests that increased D-serine metabolism resulting from increased DAAO activity may produce a reduction in NMDA receptor activity. This hypothesis was tested by Burnet et al. 2008 who examined DAAO expression and activity in postmortem cerebellar tissue from schizophrenia patients and normal controls. They reported that DAAO activity and expression were upregulated in tissue from schizophrenic patients, however, the increases in expression and activity were not related to identified SNPs or the gene products G72 and G30 [34].
In addition to human genetic studies, DAAO mutations have been studied in the mouse. ddY/DAO- mice is a naturally occurring mouse strain that lacks DAAO activity and has elevated levels of D-serine in the blood and cerebellum [35]. These mice also have functional changes in synaptic transmission including increased NMDA-mediated excitatory post synaptic currents and enhanced hippocampal long term potentiation, a molecular correlate of learning and memory [36, 37]. ddY/DAO- mice also show improved cognitive performance in the Morris water maze task36 and increased occupancy of the NMDA receptor compared to wild type animals38. Taken together, these finding suggest that DAAO inhibitors might be useful as novel therapeutics to treat psychiatric and cognitive disorders.
IN VITRO PROPERTIES AND IN VIVO EFFECTS OF DAAO INHIBITORS
Given that DAAO is involved in D-serine metabolism and that DAAO mutant mice have elevated D-serine concentration in brain, several investigators have described the use of DAAO inhibitors on D-serine levels in plasma and brain [35, 36, 38]. Thus, Adage et al. 2008 [39] described the in vitro and in vivo properties of a single compound, 5-methylpyrazole-3-carboxylic acid, ASO57278 (1. Fig. 1). No structure activity information was described however this compound was found to be a moderately potent (IC50 = 0.9µM) inhibitor of human DAAO activity in vitro. This compound was highly selective for DAAO over the glycine site of the NMDA receptor, D-aspartate oxidase (DDO) and D-serine racemase, with negligible inhibitory activity at these proteins up to 100µM. Pharmacokinetic studies of ASO57278 in rat showed it to have an intravenous half life of 5.6 h and low clearance. The compound is orally bioavailable (F=41%) with a terminal half life of 7.2 h and a Tmax of 1 h. Furthermore, the compound is brain penetrant as brain and CSF concentrations were increased following intravenous administration, although the brain to plasma and CSF to plasma ratios were relatively low at 1 h (0.052 and 0.018 respectively). AS057278 had a small but significant effect on D-serine relative to total serine (D-serine + L-serine) in the cortex and midbrain after i.v. dosing of 10 mg/kg AS057278. It is worth noting, however, that plasma levels achieved with this route and dose appeared to produce much higher drug levels than were achieved in their behavioral studies, which are described below.
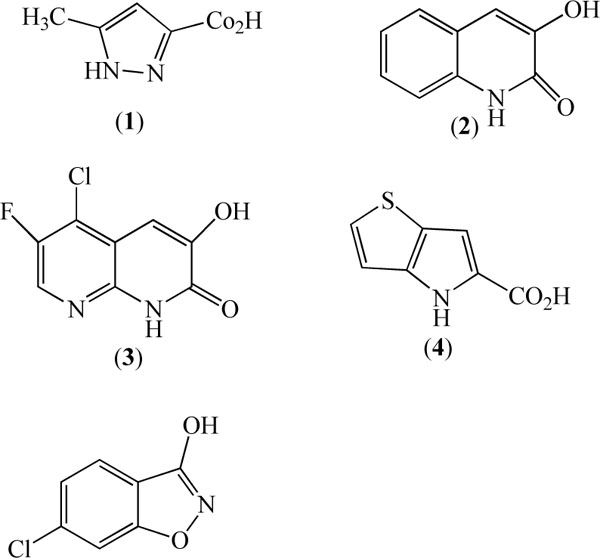
Duplantier et al. 2009 described the structure activity relationship and pharmacokinetics of 3-hydroxyquinolin-2-(1H)-one (2, Fig. 1) and its analogs as novel, selective and highly potent DAAO inhibitors [40]. Although 3-hydroxyquinolin-2-(1H)-one is a very potent DAAO inhibitor (IC50 = 4nM) it also has weak affinity for the glycine site of the NMDA receptor (29% inhibition at 10µM) and some affinity for DDO (IC50 = 855nM). Pharmacokinetic studies in rat revealed this compound to have moderate clearance (46mg/min/kg) and poor oral bioavailability (F=0.9%), but when administered subcutaneously at 56 mg/kg it increased cerebellar D-serine concentration and revealed a brain/plasma ratio of 0.7. Substitution of this compound with fluoro and chloro at positions 6 and 5 respectively and replacement of the 8 carbon atom with nitrogen culminated in an optimized compound (3, Fig. 1) that had improved oral bioavailability and maintained high affinity for rat and human DAAO (IC50 = 4nM), and weak affinity IC50 >14.5µM for DDO with negligible activity at the glycine / NMDA site. Subcutaneous administration of (3) in mice dose dependently increased cerebellar D-serine concentration 2-6 fold above control values 4 h after dosing although it is not clear if this increase of brain D-serine is due to central and / or peripheral DAAO inhibition.
Sparey et al. 2008 [41] described a two fused pyrrole carboxylic acid (4, Fig. 1) as a moderately potent inhibitor of human (IC50 = 145nM) and rat (IC50 = 112nM) DAAO in vitro with good selectivity over human DDO. The properties of (4) were further described by Smith et al. 2009 to have good rat pharmacokinetics including oral bioavailability and to dose-dependently inhibit kidney and brain (cerebellum) DAAO activity with an associated increase of plasma D-serine [42]. However, doses of (4) that inhibited DAAO activity in kidney by ~80% and in brain by ~65% failed to markedly increase cortical levels of D-serine.
Interestingly, a feature of compound 4 and several other DAAO inhibitors is the relatively small molecular weight and general lack of tolerability, in terms of maintaining high affinity, for large group substitutions. In keeping with the tolerance for small molecular weight compounds, X-ray crystallography studies indicated that the active site in DAAO is small and fairly restricted [40, 41], perhaps consistent with the physiological role of the enzyme to metabolize small molecular weight amino acids.
Ferraris et al. 2008 described a series of compounds based on a benzo[d]isoxazol-3-ol core structure as moderately potent, competitive (with respect to D-serine) inhibitors of DAAO [43]. Selectivity over DDO, glycine/NMDA or other receptors was not reported. Substitution of the core structure with larger groups (OMe, OEt, CF3) was generally poorly tolerated in terms of affinity for DAAO but smaller groups (fluoro, chloro, methyl) at positions 5 or 6 moderately improved DAAO affinity. The most potent analog described in this series was the 6-chloro analog (CBIO) which inhibited DAAO activity with an IC50 = 188nM (5, Fig. 1). Selectivity over other receptors and enzymes was not reported. While there are no reported pharmacokinetic or brain penetration studies of this compound, oral administration of CBIO to rats (30 mg/kg) increased plasma D-serine concentration similar to that produced by D-serine (30 mg/kg). Nevertheless, this dose of CBIO failed to increase extracellular cortical D-serine concentrations when administered alone and a similar lack of effect of CBIO on rat brain D-serine was found by Hashimoto et al. 2009 [43, 44].
Collectively, the limited experience with a small number of structurally diverse inhibitors indicates that extensive inhibition of peripheral and central DAAO has a limited effect on brain or extracellular D-serine concentration. This could be due to several reasons. High affinity D-amino acid reuptake sites could keep extracellular D-serine concentrations low even when DAAO activity is inhibited. Regional differences in the activity of brain DAAO could be a source of error in assessing the efficacy of inhibitors (cortex has very low DAAO activity compared with cerebellum). Poor brain penetration or lack of interaction of the inhibitor with the enzyme in situ. In this regard, the paper by Duplantier et al 2009, indicated that free compound in brain may need to be several-fold greater than the in vitro IC50 before significant elevations in D-serine are observed, at least in the cerebellum which has a high level of DAAO activity [40].
EFFECT OF DAAO INHIBITORS ON BEHAVIORS RELEVANT TO SCHIZOPHRENIA
When co-administered with antipsychotics, D-serine, as well as direct administration of other co-agonists of the NMDA receptor, has been reported to have therapeutic effects in patients with schizophrenia [29 – 32]. For this reason, several reports have investigated the effects of D-serine administration in preclinical models and have demonstrated effects in assays predictive of clinical utility for positive symptoms [42, 45, 46] negative symptoms [21] and cognitiom [42, 47, 48].
In contrast to the fairly robust effects reported with D-serine administration, the reported behavioral effects of DAAO inhibitors are fairly modest and inconsistent. For example, we found that D-serine attenuated the psychomotor activating and dopamine releasing effects of amphetamine and reversed an MK-801 induced deficit in novel object recognition. In contrast, compound (4) did not produce behavioral or neurochemical changes in these assays. In addition, we have hitherto unpublished data showing that D-serine improves recognition in a time-dependent forgetting protocol to assess novel object recognition, whereas compound (4) does not (Fig. 2). Importantly, we found that the dose of D-serine required for improvement in novel object recognition and attenuation of amphetamine-induced psychomotor activity elevated CSF D-serine 40-fold over that achieved by the maximum dose of compound (4) tested (200 mg/kg). These findings suggest that the increase in D-serine needed for these behavioral effects is much greater than can be achieved by DAAO inhibition, at least by a single dose of compound (4). Administration of the DAAO inhibitor CBIO on its own also reportedly failed to reverse a prepulse inhibition (PPI) deficit induced by MK-801 administration whereas D-serine was effective [44].
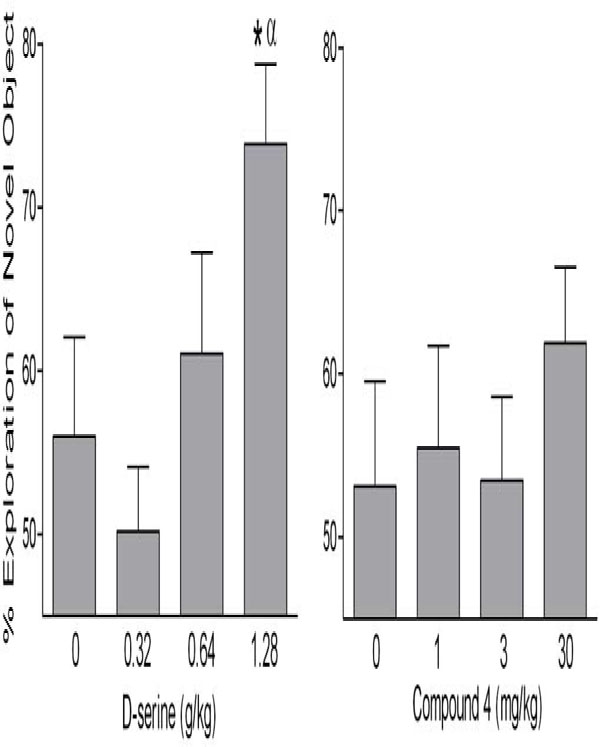
The influence of D-serine and compound 4 on novel object recognition. Groups of male Wistar Hannover rats were given D-serine (s.c.) or compound 4 (i.p.) and 4 hours later were placed in test cages and allowed to explore two identical objects for 90 seconds. 24 hours later these animals were placed back in the test cage and allowed to explore one object they had explored previously and one novel object. Memory for the previously encountered object is indicated by greater exploration of the novel object. The group given 1.28 g/kg d-serine showed greater object recognition than the vehicle treated animals, as well as greater than chance levels of exploration of the novel object. In contrast, none of the doses of compound 4 increased recognition. * indicates significantly greater % exploration of the novel object, relative to 0 mg/kg. α indicates greater than chance (50%) levels of exploration of the novel object.
In contrast to the negative results described above, Adage et al., 2008 found that acute administration of 80 mg/kg AS057278 reversed a PCP-induced deficit in PPI, although the effect was not observed with other doses, including lower (40 mg/kg) or slightly higher (100 mg/kg) doses of AS057278. These authors also report that chronic (28 days, b.i.d.) administration of AS057278 reversed a PCP-induced deficit in PPI, but again the dose-effect curve followed a steep inverted U-shaped dose-effect function (10 and 40 mg/kg were ineffective, whereas 20 mg/kg was effective). Finally, these authors reported that subchronic, but not acute administration of AS057278 attenuated the psychomotor activating effects of PCP. Unfortunately, no measurement of brain D-serine was made after chronic dosing to determine if the more pronounced behavioral effects of AS057278 following chronic dosing were due to elevated D-serine.
Given the moderate efficacy of DAAO inhibitors on brain D-serine and behavior, several authors have investigated the effects of co-administering DAAO inhibitors with D-serine on brain neurochemistry and behavioral assays. Indeed, Ferrais et al, 2008 showed that CBIO had quite pronounced effects on brain (and plasma) D-serine when co-administered with 30 mg/kg D-serine, relative to when either CBIO or D-serine was administered alone. Hashimoto et al, 2009 extended this finding by showing effects on cortical D-serine and also demonstrated that co-administration of D-serine (30 mg/kg) and CBIO reversed an MK-801-induced deficit in PPI whereas the 30 mg/kg dose of D-serine had no effect on its own. We have likewise shown that co-administration of compound (4) with D-serine elevates CSF and cortical D-serine to a greater extent than administration of D-serine alone in male rats (Fig. 3). Unfortunately it is not known whether the effects of CBIO or compound (4) on elevating D-serine levels following D-serine administration are due to inhibition of DAAO in the periphery, brain or most likely both compartments. Neither is it clear from any of these studies whether or not such interactions are synergistic or additive and more extensive isobolargraphic analyses would be required to define the interaction. Nevertheless, these findings suggest that DAAO inhibitors could be useful clinically for reducing the dose of D-serine necessary to improve psychosis or cognitive deficits associated with schizophrenia. As a result, the co-administration of DAAO inhibitors with D-serine could ameliorate some of the side effects associated with the administration of high doses of D-serine, such as nephrotoxicity [49].
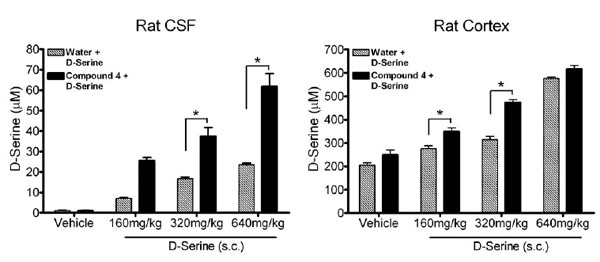
Effects of compound 4 on CNS D-serine concentration following D-serine administration. CSF levels of D-serine 4h following D-serine administration in animals pretreated (30 min) with compound 4 (25 mg/kg, i.p.) or water (2 ml/kg, i.p.) (left). Cortical levels of D-serine 4h following D-serine administration in animals pretreated with compound 4 or water (right). * indicates a significant increase in D-serine in compound 4 pretreated animals compared to vehicle pretreatment (p< 0.05, ANOVA). Data reported as mean ± SEM, n=5 per group.
OTHER THERAPEUTIC INDICATIONS FOR DAAO INHIBITORS
Beyond its hypothesized involvement in schizophrenia, there is also evidence suggesting a role of D-serine, and potentially DAAO, in the response to painful stimuli. Intracerebral administered D-serine is effective in several pain models [50, 51] although the effects of D-serine on pain responsiveness might greatly differ depending on the brain region where it is administered (see [52] for an example of potentiation of the pain response by D-serine). However, there is a significant amount of research showing that NMDA receptor antagonists might be also useful for treating pain [53], so the mechanism of action by which D-serine has reported analgesic effects is not well understood.
The effect of DAAO inhibition on the response to painful stimuli has not been well studied and the few relevant studies are somewhat contradictory. Ren et al, 2006 showed that infusion of the enzyme DAAO into the rostral anterior cingulate cortex reduced conditioned place avoidance produced by formalin injections, but had no effect on acute pain responses induced by formalin [54]. These results were interpreted to indicate that decreasing D-serine (and presumably other D-amino acids) in the anterior cingulate via upregulating DAAO produces analgesic effects, and might suggest a nociceptive effect of inhibiting the enzyme. Indeed, Wake et al (2001) showed that a mutant mouse lacking DAAO activity is supersensitive to formalin-induced nociception [37]. On the other hand, Zhao et al, 2008 showed that this same mouse strain is less sensitive to a variety of painful stimuli (including formalin) and that the DAAO inhibitor sodium benzoate has analgesic effects in two chronic pain assays [55]. These inconsistent findings highlight the need for more studies to address the potential of DAAO inhibitors for treating pain. Nevertheless, Sepracor has revealed that they plan on evaluating the effects of their DAAO inhibitor SEP-227900 for neuropathic pain.
CONCLUSIONS
The therapeutic potential of DAAO inhibitors is relatively unexplored and studies have primarily addressed their value for pharmacotherapy in the treatment of positive, negative and cognitive symptoms of schizophrenia. However, the few published studies characterizing novel DAAO inhibitors have yielded conflicting results. This may be for several reasons including the use of DAAO inhibitors with different properties including potency and pharmacokinetics. Relatively few of the published studies have related efficacy (or lack thereof) to the extent of peripheral / brain DAAO inhibition or have demonstrated an increase of brain extracellular D-serine following a behaviorally effective dose of an inhibitor. Furthermore, the relative contributions of peripheral D-serine which can be actively transported into the brain are poorly understood. Interestingly, preclinical studies have provided data that combining a DAAO inhibitor with D-serine may be more effective in terms of antipsychotic like activity, however, a clinically acceptable strategy for this combination remains to be determined.


