All published articles of this journal are available on ScienceDirect.
Exploration of Adhesion Molecule Expression in Cardiac Muscle of Early Atherosclerosis Dyslipidemic Sprague Dawley Rats
Abstract
Objective:
This study is aimed to examine the expression of ICAM-1 and VCAM-1 in cardiac tissue of dyslipidemic Sprague Dawley rats.
Methods:
Eight Sprague Dawley strain rats, with 150-200 gram body weight, were divided into two groups. The control group was fed a standard diet, the positive control group was fed a high-fat diet as our previous study for 8 weeks. The pattern of distribution of ICAM-1 and VCAM-1 in cardiac muscle cell was examined by immunofluorescence and observed with a confocal laser scanning microscope. Lipid profile was also examined at the end of the study.
Result:
Independent t-test showed no differences in ICAM-1 and VCAM-1 expression in cardiac muscle of hypercholesterol-diet-fed Sprague Dawley rat compared to control.
Conclusion:
The expression of ICAM-1 and VCAM-1 in cardiac muscle did not change after the onset of atherosclerosis.
1. INTRODUCTION
Ischemic heart disease and stroke recently have been known as the leading cause of death in the world (World Health Organization, 2014) [1-3].Stroke occurs in 1 out of 20 causes of death in US every year (American Heart Association, 2015). Additionally, stroke also becomes the most leading causes for long-term disability [3]. Cardiovascular disease and stroke are caused by several risk factors such as smoking, physical inactivity,obesity, hypertension, diabetes, and dyslipidemia [4].
Dyslipidemia is established as a major risk factor for CVD and stroke [5]. Dyslipidemia is a lipid metabolic impairment condition caused by interaction between genetic and environmental factors, which increases accumulation of Low-Density Lipoprotein (LDL) in the blood. In endothelial cells, LDL undergoes modification and becomes Ox-LDL, as a result of macrophage and endothelial cells activities. A lipid that accumulates in the endothelium becomes the basis for endothelial dysfunction leading to the formation of atherosclerosis [6].
Recently one of emerging theories beyond the formation of atherosclerosis that is widely studied is the involvement of lipoprotein-associated phospholipase A2 (Lp-PLA2) [7-9]. Lp-PLA2 is divided into secreted Lp-PLA2 and found in the circulating system and latent Lp-PLA2 located within atherosclerotic plaque. Approximately 70% of secreted Lp-PLA2 binds to LDL-C and hydrolize LDL into lysophosphatidylcholine (Lyso-PC). While Lp-PLA2 in atherosclerotic plaque hydrolize oxidized LDL (oxLDL) into Lyso-PC and oxidized non-Esterified fatty acid (Ox-NEFA)9. Lyso-PC and Ox-NEFA then stimulate the expression of adhesion molecules, upregulate inflammatory cytokines, enhance matrix metalloproteinase expression, amplify oxidation and expand necrotic lipid core and thinning fibrous cap [10].
Intracellular cell adhesion molecule-1 (ICAM-1) and Vascular cell adhesion molecule-1 (VCAM-1) are immunoglobulin superfamily members that play important roles in adhesion of leukocytes to vascular endothelium [11]. ICAM-1 has been known to be upregulated at the onset of pressure overload by aortic constriction and in the presence of cardiac inflammation mediates T-cell recruitment during pathologic cardiac remodelling in heart failure [12-14]. VCAM-1 is strongly upregulated in the heart during acute and chronic stages of diseases such as in inflammatory cardiomyopathy [15]. This study was aimed to explore ICAM-1 and VCAM-1 expression of cardiac tissue from the early progression of atherosclerosis in dyslipidemia in Sprague Dawley rat model.
2. MATERIALS AND METHODS
2.1. Study Design
Eight Sprague Dawley strain rats, 6-8 weeks old, with 150-200 gram body weight, were obtained from Bogor Agricultural University, Indonesia. Rats were divided into two groups after acclimatization. The control group was fed a standard diet (N), the positive control group was fed a High-Fat Diet (HFD) as our previous study [16] (DL) for 8 weeks. This study was conducted at the Biomedical Laboratory and Central Laboratory of Biological Sciences, Brawijaya University after obtaining ethical clearance assessment by the Health Research Ethics Committee.
2.2. ICAM-1, and VCAM-1 Measurement
Heart organ was separated from the pulmonary artery, pulmonary vein, aorta, and vena cava fixed with PHEMO buffer (68 mM PIPES, 25 mM, HEPES, pH 6.9, 15 mM EGTA, 3 mM MgCl2, 10% [v/v] dimethyl sulfoxide containing 3.7% formaldehyde and 0.05% glutaraldehyde). Each parameter was then processed for imumunofluoresence with VCAM-1 anti-rat antibody using fluorescein isothiocyanate secondary antibody and ICAM-1 anti-rat antibody using rhodamin secondary antibody (BIOS Inc., Boston, MA, USA). The luminescences were observed with confocal laser scanning microscopy (Olympus Corporation, Tokyo, Japan) and were quantitatively analyzed by Olympus FluoView software (version 1.7A; Olympus Corporation) Fig. (1).
3. RESULT AND DISCUSSION
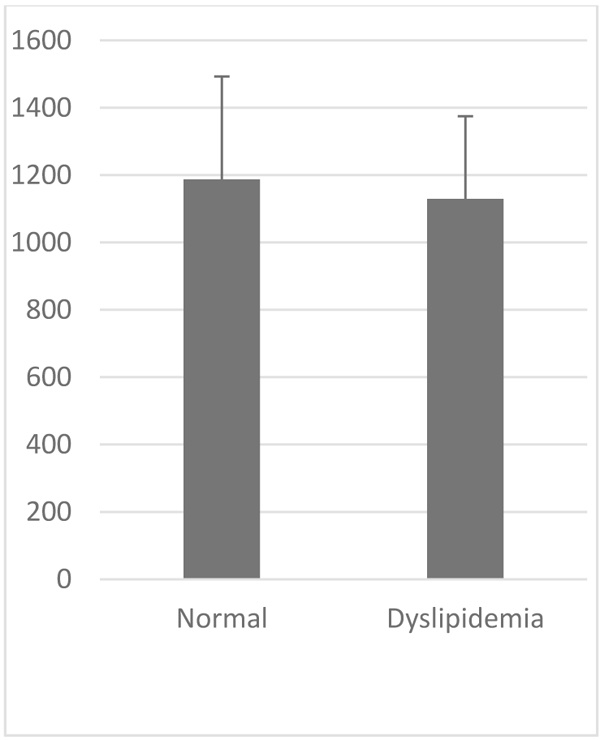
VCAM-1 expression in the normal group was similar to that of high-fat diet group (Table 1, Figs. 2 and 3). Likewise, the expression of ICAM-1 in the normal group was not significantly different compared to the high-fat diet group.
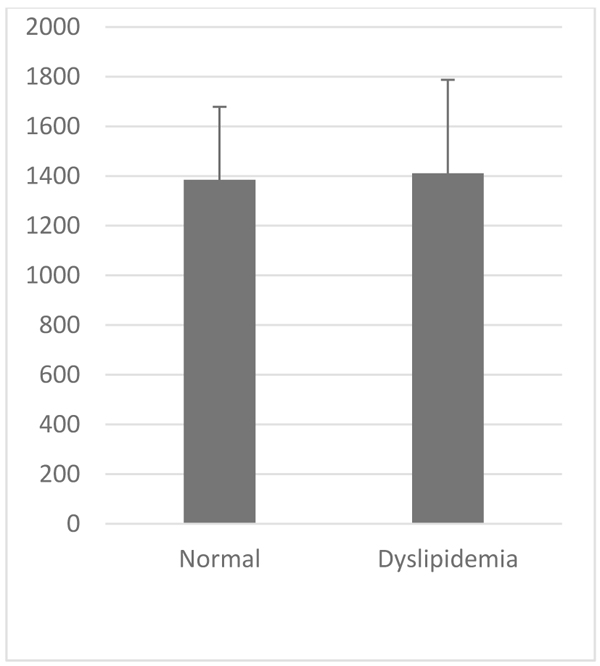
| Parameter | VCAM-1 (au) | ICAM-1 (au) | |
|---|---|---|---|
|
Normal 8 Week (Mean±SD) |
1187.49±305,33 | 1384.89±293,59 | |
|
Dyslipidemia 8 Week (Mean±SD) |
1129,47±245,539 | 1411,05±375,94 | |
| P Value | >0.05 | >0.05 | |
High fat diet induces abnormal amount of lipids in the blood or dyslipidemia causing hyperlipidemia which is characterized by elevation of total cholesterol and/or elevation of low-density lipoprotein (LDL) cholesterol and/or elevation of triglyceride concentrations and/or decrease of high-density lipoprotein (HDL) cholesterol in the blood [17]. Lipid profile result from our previous publication was in line with this theory [18]. Lipid is normally stored in adipose tissue as an esterified lipid, but in high-fat diet, 40%-50% of lipid undergoes spillover causing ectopic fat deposition [23]. Fat deposition in blood vessels causes infiltration of apoB containing lipoproteins (LDL, remnant VLDL, and chylomicrone) in the artery wall. The retained lipoproteins induce ROS production that lead to endothelial dysfunction. ROS then modify LDL into oxLDL and oxLDL which then induce more ROS formation [19].
Oxidative stress has an important role in the pathophysiology of atherosclerosis. Sufficient levels of ROS, particularly superoxide anion and hydrogen peroxide have been shown to activate nuclear factor kappa beta (NF-κB) [20]. NF-kB are transcription factors which bind to VCAM-1 and ICAM-1 promoter and induce VCAM-1 and ICAM-1 gene transcription. This process promotes the binding, rolling and stable arrest of inflammatory white blood cells, such as T cells, monocytes and mast cells [21]. Cytokine production by inflammatory cells within endothelial like TNF-α and IL-1 also works on endothelial cell and induces endothelial cells to express VCAM-1 and ICAM-1 via NF-κB activation. ICAM-1 and VCAM-1 play a major role in the initiation of early atherosclerosis, mainly its contribution to monocyte adhesion. Inhibition of the inflammatory response is widely known to be beneficial in the early stages of atherosclerosis [22].
CONCLUSION
Results from our study show that there were no differences between ICAM-1 expression in dyslipidemia group compared to the normal group and no differences were observed between VCAM-1 expression in dyslipidemia group compared to the normal group. ICAM-1 and VCAM-1 in aorta may have an important role in the initiation of atherogenesis, while in cardiac tissue, ICAM-1 and VCAM-1 are reported to be upregulated in several studies at the onset of pressure overload by aortic constriction and in the presence of cardiac inflammation such as in myocardial infarction or inflammatory cardiomyopathy [12-15]. Soluble VCAM-1 also is an established biomarker to predict future or long-term acute coronary syndrome [23-25]. While the cardiac effect of atherosclerosis is not directly due to atherosclerosis in itself, the cardiac effect of atherosclerosis is due to the inflammatory response of an infarct due to aortic constriction or coronary artery constriction. Therefore, adhesion molecule expression ICAM-1 and VCAM-1 was not upregulated in the early progression of atherosclerosis and they are less likely to contribute to cardiomyopathy because of dyslipidemia.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Animal Care and Use Committee of Brawijaya University, No: 229-Kep-UB.
HUMAN AND ANIMAL RIGHTS
Humans did not participate in this research. All animal research procedures followed were in accordance with the US National Research Council's “Guide for the Care and Use of Laboratory Animals,”
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This study was funded by Indonesia Ministry of Research, Technology, and Higher Education with grant No. 530.2/UN10.21/PG/2015.


