All published articles of this journal are available on ScienceDirect.
Anti-diabetic Effect of Acridocarpus Orientalis
Abstract
Background:
Acridocarpus orientalis (AO) is a medicinal herb indigenous to tropical and subtropical Africa, Arabian Peninsula, and New Caledonia with reported anti-inflammatory and antioxidant properties.
Objective:
To determine whether AO has any beneficial effects on diabetes-induced metabolic parameters in rats.
Materials and Methods:
Diabetes mellitus was induced in male Wistar rats by streptozotocin. Diabetic rats were treated with three doses of AO extract (50, 100, and 200 mg/kg BW) for 30 days. Kidney, liver, and pancreatic tissue samples were processed for histopathology to determine the effect of AO on the cells of these organs. The effect of AO on pancreatic islet cells and serum insulin levels was also examined using immunohistochemistry and enzyme-linked immunosorbent assay techniques, respectively.
Results:
AO (100 mg/kg BW) caused a marked reduction in blood glucose levels in diabetic rats compared to diabetic control on day 10 of the study. Moreover, AO (200 mg/kg BW) increased the number of insulin-positive cells with a concomitant reduction in the number of glucagon-immunoreactive cells in pancreatic islets. AO (100 mg/kg) also increased the serum level of superoxide dismutase significantly. Although the administration of AO was able to significantly decrease the diabetes-associated increases in serum creatinine and bilirubin levels, it had no effect on blood urea nitrogen, serum aspartate, or alanine aminotransferase levels. Histopathological examination showed that AO has no toxic effect on the structure of the pancreas, liver, and kidney.
Conclusion:
Our findings showed that AO could alleviate some complications of diabetes mellitus.
1. INTRODUCTION
Diabetes Mellitus (DM) is a chronic endocrine disease with a high prevalence in urban populations. The number of people with DM is expected to reach 552 million cases by the year 2030, according to the International Diabetes Federation [1]. DM is caused by several genetic and environmental factors. It is associated with high blood glucose levels because of dysfunctional insulin secretion or cellular resistance to insulin binding [2]. Three main types of DM have been identified. Type 1 DM is associated with the near destruction of pancreatic beta cells of the endocrine pancreas, leading to the secretion of a low and inadequate quantity of insulin. In contrast, type 2 DM is associated with a sluggish lifestyle, unhealthy diet, obesity, adverse environmental factors such as drugs, viral infection, and genetic disorders [3, 4]. The other major type of DM is referred to as gestational diabetes, which occurs in a subtle form during pregnancy. Women with gestational DM may eventually develop overt DM after parturition [5]. Chronic untreated hyperglycemia induces oxidative stress [6], leading to the development of many macro- and micro-vascular complications such as cardiac diseases, retinopathy, neuropathy, and nephropathy [7-9]. All of these complications play a role in the high morbidity and mortality seen in patients with DM [10]. The short and long-term complications of DM and the associated signs and symptoms have indeed created a severe burden on health care providers worldwide [10]. Since the prevalence of DM will continue to rise as reported in a previous study [1], the need to manage DM, and its complications becomes even more important.
In addition, the need to discover a less expensive way in the treatment of DM fits into this endeavor. Herbal medicine is cost effective and it is one of the oldest traditional ways for the treatment of many diseases, including DM. Herbal medicine uses a natural source of medication for the treatment of diseases with the added advantage of easy availability and simplicity. Many of the herbs can be easily cultivated. Some of the herbs may come with reduced side effects when compared to pharmaceutical drugs. It has been estimated that about 80 percent of people worldwide are using herbal medicine in curing a variety of diseases [11]. Moreover, a large number of pharmaceutical drugs actually originated from natural plants [12].
Acridocarpus orientalis (AO) A. Juss (genus: Acridocarpus; family: Malpighiaceae) is one of the folk medicinal plants that have been used in the treatment of many diseases, such as cancer. AO is one of several herbs used to treat various illnesses in the Middle East. Although it has been reported that AO has anti-inflammatory and antioxidant properties [13], the effect of AO on a metabolic disease such as diabetes mellitus has not been investigated. AO is a flowering tropical plant, found in Africa, Asia and the Middle East.
The phytochemical analysis of AO extracts showed that it has many ingredients, such as; tannins, phenols, saponins and flavonoids compounds with antioxidant activities [13, 14].
The role of AO in mitigating the signs and symptoms of diabetes mellitus has not been elucidated. Since it is also well known that some herbal medication may cause harmful side effects [15] than curing the disease itself, it is important to determine whether a given herbal plant is toxic to major parenchymal organs such as the liver, kidney, and pancreas. Since the liver is the detoxification center of the body [16], an ideal drug, therefore, whether it is a herb or a synthetic preparation, should not be toxic to the liver or cause hepatic pathology that will effectively incapacitate its function.
In the present study, we hypothesized that the ethanol extract of AO increases the number of pancreatic beta cells, body weight, insulin level and reduces hyperglycemia in an animal model of diabetes. This hypothesis is based on reports that AO contains a high level of the plant antioxidant, polyphenols [13]. Recent reports have also shown that AO contains morin, a flavonol antioxidant [14]. We also wanted to clarify whether AO causes histopathological changes or has protective effects in the liver, kidney, and pancreas after the onset of diabetes.
2. MATERIALS AND METHODS
2.1. Reagents and Antibodies
Streptozotocin was purchased from Sigma-Aldrich (MO, USA; purity: ≥ 98% by HPLC). Insulin and glucagon primary and their respective secondary antibodies were purchased from Santa Cruz Biotechnology (CA, USA). Rat insulin ultrasensitive ELISA kits are products of Millipore (MO, USA).
2.2. Experimental Animals
In this study, 3 month-old male Wistar rats weighing 250 g and bred at the College of Medicine and Health Sciences (CMHS), United Arab Emirates University, were used. All rats were housed at a temperature of 25oC and humidity-regulated animal facility with a cycle of 12 h light/12 h darkness. The animals received rodent feed and water ad libitum. The CMHS’s Animal Research Group guidelines for the care and use of laboratory animals were followed (Ethical clearance number: A2-R07).
2.3. Rodent Model of Diabetes Mellitus
Diabetes mellitus (DM) was induced in male Wistar rats by a single intraperitoneal administration of streptozotocin (STZ, Sigma-Aldrich, MO, USA) at 60 mg/kg body weight [17, 18]. Age- and gender-matched, non-diabetic control Wistar rats were treated with citrate buffer solution only. Blood glucose levels were tested on the tail vein using Optium Xceed Glucometer (Abbott Laboratories, IL, USA) five days after treatment with either STZ or citrate buffer solution. Animals with a ≥ 300 mg/dl fasting blood glucose level were selected for AO treated and untreated diabetic groups. Diabetic (n=6) and non-diabetic control (normal, n=6) rats received daily oral gavage of Acridocarpus orientalis extracts of either 50, 100, or 200 mg/kg body weight for 30 days. In addition, one diabetic group received a daily dose of glibenclamide at 0.25 mg/kg body weight (BW) according to a previously reported dosage regimen [19]. Two other groups, untreated diabetic and normal control rats, were treated with only saline solution for the same duration of time. The total body weight and fasting blood glucose level of each rat for each group were recorded every 10 days. All measurements except BW and fasting blood glucose level were taken at the end of the experimental period.
2.4. Plant Material and Preparation of Extract
Aerial samples of AO (Acridocarpus orientalis) A. Juss. (Genus: Acridocarpus, Family: Malpighiaceae, local name: qafas) were collected from Jabal Hafeet (Al Ain, UAE, N 24.19, E 55.62), washed, air-dried and then stored in darkness at room temperature. The aerial samples retrieved were verified and deposited at the Herbarium of the Department of Biology, College of Science, UAE University, Al Ain. The aerial samples of AO were crushed, filtered, and the components were extracted three times with fresh ethanol at room temperature for a period of 12 h. The ethanolic phase of the extraction was recovered, dried at 40oC by rotary evaporator, and lyophilized according to a previously reported method [13]. Lyophilized extracts were stored in the dark at 4oC.
2.5. Body Weight, Fasting Blood Glucose Measurement and Intraperitoneal Glucose Tolerance Test
The weight of normal and diabetic rats was measured every 10 days using the Sartorius scale (Goettingen, Germany). The fasting blood glucose level in the tail vein was recorded every ten days for each individual rat in all groups after an overnight fast.
Intraperitoneal Glucose Tolerance Test (IPGTT) was performed in normal and diabetic rats at the end of the experimental period after fasting the animals for 18 h. Each rat from all of the four groups was given an intraperitoneal glucose load of 2 g/kg BW according to a previously published procedure [20]. Blood glucose levels were taken at zero time (before glucose load), 30, 60, and 120 min after intraperitoneally delivered glucose load.
2.6. Tissue and Blood Collection and Tissue Processing
At the end of the experiment, all rats from all groups were subjected to general anesthesia using diethyl ether. Following decapitation, whole blood was collected into vacutainer tubes. The pancreas, liver, and kidney tissue samples were expeditiously removed, and stored for pathohistological investigations according to a previously reported method [21].
2.7. Light Microscopy of the Pancreas, Liver and Kidney
The isolated pancreatic, liver and kidney tissue fragments from each rat in each group were trimmed free of connective tissues and processed for hematoxylin and eosin (H & E) staining according to previously reported methods [22, 23].
2.8. Immunofluorescence Study
Pancreatic tissue samples from treated and untreated normal and diabetic rats were trimmed free of connective tissues and processed for immunohistochemistry using a previously described method [18], employing antibodies against insulin and glucagon. Briefly, pancreatic tissue fragments were fixed, embedded in liquid paraffin and later processed for insulin and glucagon double-labelling immunofluorescence study according to a previously described technique [20, 24], using antibodies against insulin and glucagon.
2.9. Morphometric Analysis of Pancreatic Islet Cells
The percentage distribution of pancreatic alpha (glucagon-positive) and beta (insulin-positive) cells was examined using Image J Software (NIH, MA, USA). We counted the total number of either insulin or glucagon positive cells compared to the total number of cells in the whole islet in accordance with a previously reported morphometric method [18].
2.10. Serum Insulin
The serum insulin level was determined by using a commercial kit (Millipore, MO, USA). All values were expressed as ng/ml. Samples were prepared for the determination of insulin using methods reported by Adeghate et al. [25].
2.11. Measurement of Antioxidant Activity
The activity of the anti-oxidative enzyme, superoxide dismutase (SOD), which converts superoxide anion (O2.-) into hydrogen peroxide and harmless molecular oxygen, was measured using SOD Assay Kit-WST (Sigma-Aldrich, MO, USA). Measurement of antioxidant activity was performed according to a recently reported method [26].
2.12. Biochemical Analysis
Serum levels of the following parameters were determined using Cobas biochemical analyzer (Roche, Rotkreuz, Switzerland): aspartate aminotransferase, alanine aminotransferase, creatinine, bilirubin, creatinine, and blood urea nitrogen [27].
2.13. Statistical Analysis
All values were calculated as mean ± standard error of the mean (SEM). ANOVA test was used to determine the significance between different groups. SPSS Statistical Analysis Software was used for statistical analysis. A p value of ≤ 0.05 was taken as a reference for significance.
3. RESULTS
3.1. Body Weight, Glucose Level and Glucose Handling
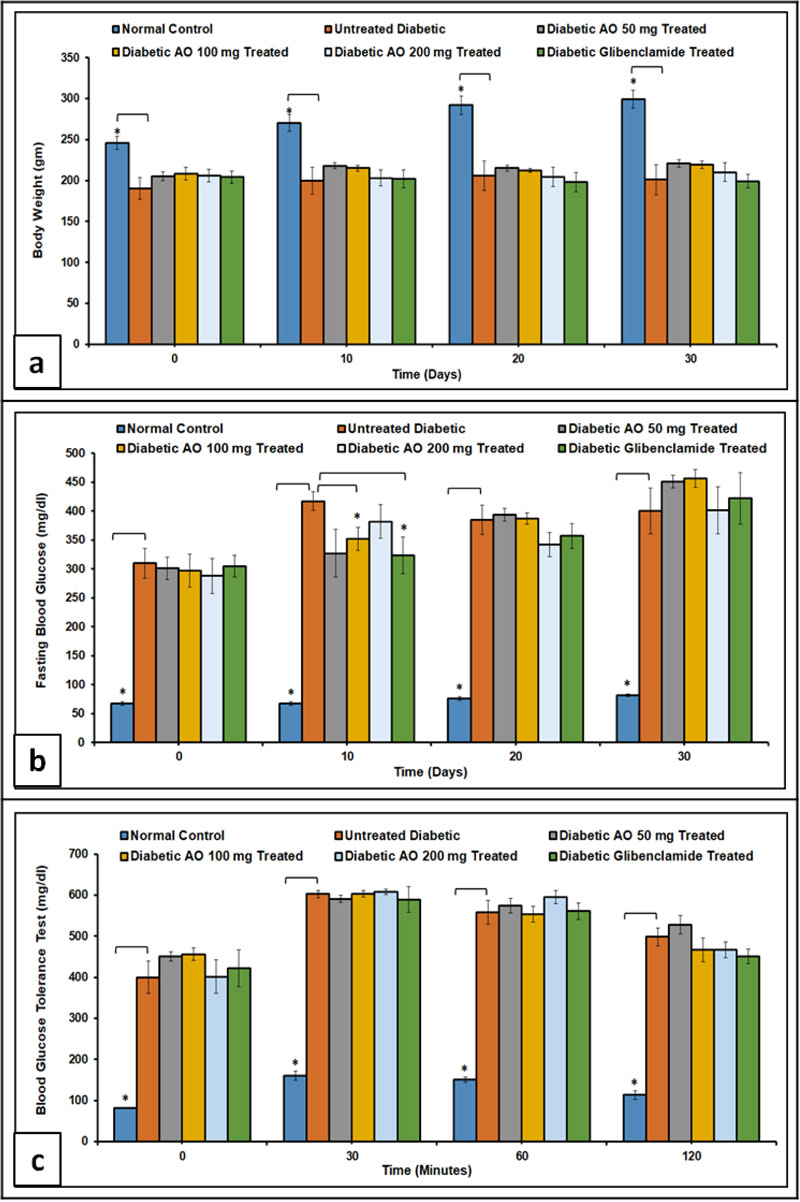
Normal control rats showed a remarkable weight gain (p < 0.05) when compared to AO- and glibenclamide-treated and untreated diabetic rats (Fig. 1a). The weight gain observed in treated diabetic groups was not significantly different 30 days after the induction of diabetes. Fasting blood glucose level was significantly (p < 0.05) higher in treated and untreated diabetic rats compared to non-diabetic controls. It is worth noting, however, that diabetic rats treated with either a 100 mg/kg body weight of AO or glibenclamide (0.25 mg/kg body weight) showed a significant (p < 0.05) reduction in blood glucose level 10 days after the induction of diabetes when compared to untreated diabetic rats (Fig. 1b).
Intraperitoneal Glucose Tolerance Test (IPGTT) showed that blood glucose levels of treated and untreated diabetic rats at 0, 30, 60, and 120 min after glucose load were markedly (p < 0.05) higher when compared to non-diabetic control (Fig. 1c).
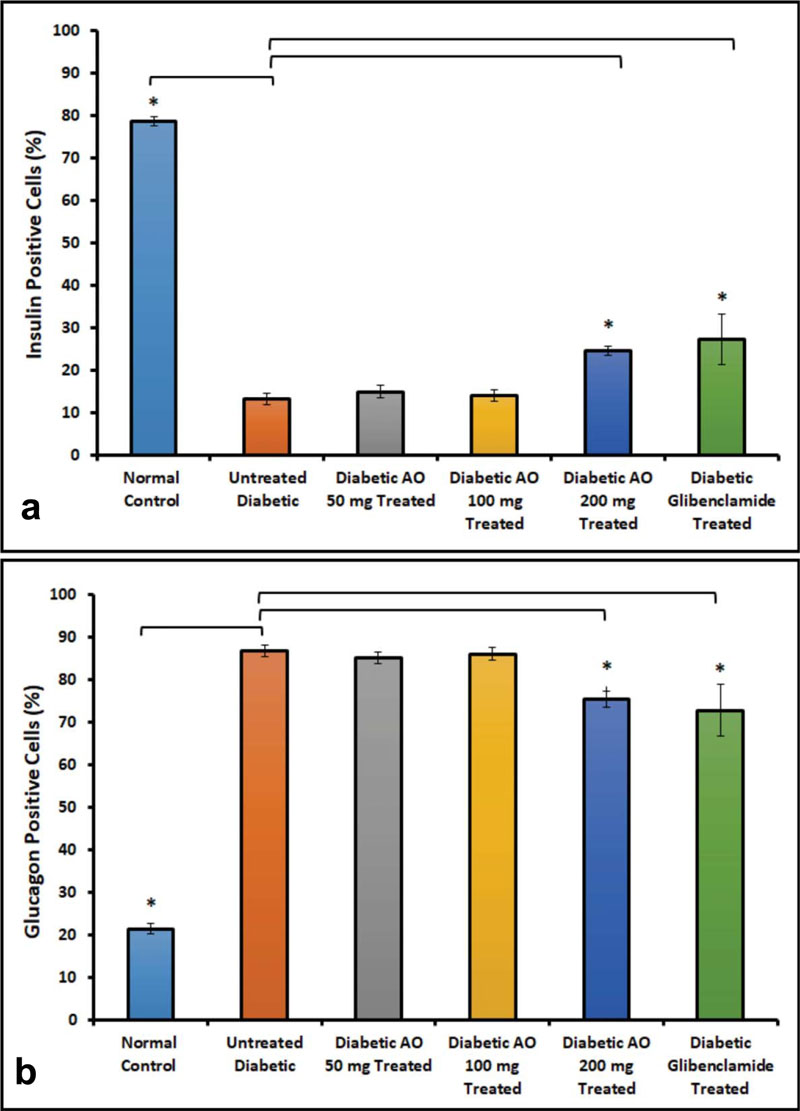
3.2. Distribution of Insulin- and Glucagon-positive Cells in Pancreatic Islets
Double-labelling immunofluorescence method was used to determine the localization of insulin-positive and glucagon-positive cells in the islets of normal, treated and untreated diabetic rats (Fig. 2). The results showed that insulin-positive cells are located in the central part of the islet of normal control with glucagon-immunoreactive cells placed peripherally. The islets of untreated diabetic rats contain fewer insulin-positive cells but more of glucagon-positive cells when compared to AO-treated rats. It is worth noting that the number of insulin-positive cells increased significantly (p < 0.05) with a concomitant reduction in the number of glucagon-producing alpha cells in diabetic rats treated with either AO (100, 200 mg/kg body weight) or glibenclamide (0.25 mg/kg body weight) when compared with untreated diabetic rats (Fig. 3).

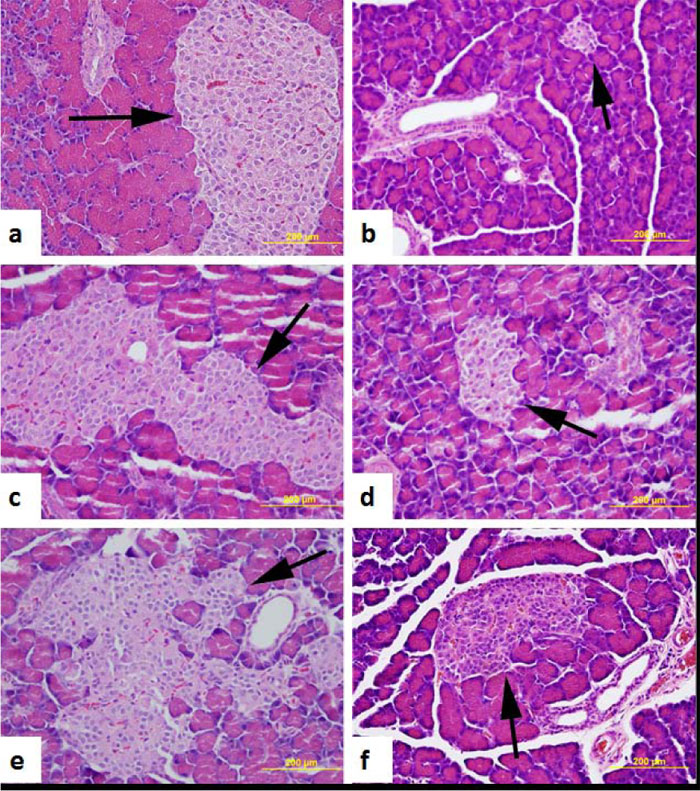
3.3. Morphology of the Pancreas, Liver, and Kidney
The histology of H & E stained pancreatic rats islets of either normal control or AO (50, 100 and 200 mg/kg body weight) or glibenclamide (0.25 mg/kg body weight) treated diabetic rats is displayed in Fig. (4). The structure of the exocrine and endocrine components of the pancreas appears to be intact in normal and in treated, compared to untreated dia- betic rats. The number and size of pancreatic islets are significantly smaller in the pancreas of untreated diabetic rats compared to normal and AO-treated rats (Fig. 4).
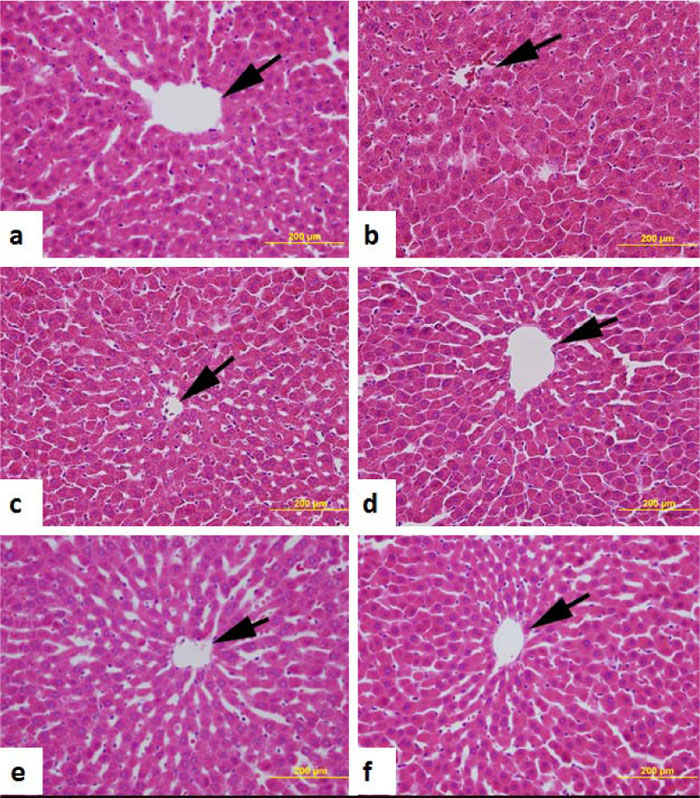
Liver sections showed normal liver cell morphology forming hepatic lobules with radiating cords of hepatocytes from the central vein in both control and AO-treated and untreated diabetic rats (Fig. 5).
Morphology of the kidney of normal control, untreated diabetic and those of diabetic rats treated with either AO (50, 100 and 200 mg/kg body weight) or glibenclamide (0.25 mg/kg body weight) is shown in Fig. (6). Normal control rats, untreated diabetic rats, and diabetic rats treated with AO or glibenclamide showed almost normal kidney histology.
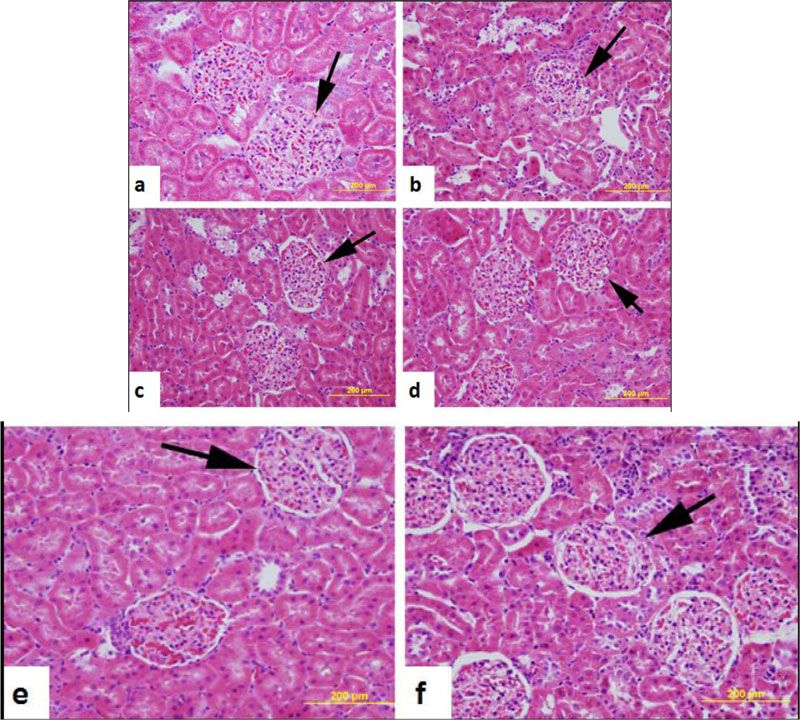
3.4. Liver and Kidney Functions Tests
The effect of either AO (50, 100 & 200 mg/kg body weight) or glibenclamide (0.25 mg/kg BW) treatments on serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) is shown in Figs. (7a) and (b). No significant change in the levels of AST and ALT between normal and AO-treated and untreated rats was observed. In contrast, there was a significant reduction in total serum bilirubin levels in diabetic rats treated with either AO (100 and 200 mg/kg BW) or glibenclamide (0.25 mg/kg BW) (Fig. 7c).
The effect of AO (50, 100 and 200 mg/kg BW) and glibenclamide (0.25 mg/kg BW) treatment on serum creatinine in normal, treated and untreated diabetic rats is shown in Fig. (7d). The data demonstrated that the treatment of diabetic rats with AO at 200 mg/kg BW) significantly (p < 0.05) reduced serum creatinine when compared to untreated diabetic rats. The blood urea nitrogen (BUN) level is significantly higher (p < 0.05) in diabetic rats compared to non-diabetic control. BUN level failed to change significantly after treatment with either AO or glibenclamide (Fig. 7e).
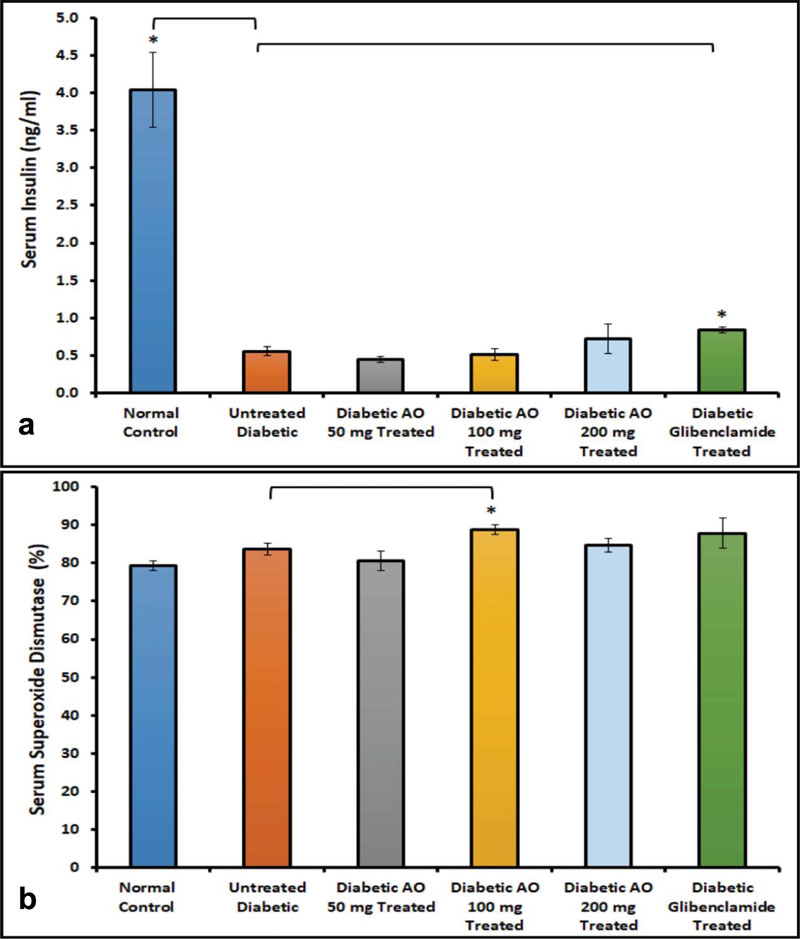
3.5. Serum Insulin and SOD
The effect of either AO or glibenclamide treatments on serum insulin levels in diabetic rats was investigated to determine whether AO has a secretagogue effect. The results indicated that serum insulin concentration was significantly (p < 0.05) elevated in glibenclamide-treated, but not in AO-treated diabetic rats, compared to untreated diabetic rats (Fig. 8a).
We also examine the effect of AO (50, 100 & 200 mg/kg BW) and glibenclamide (0.25 mg/kg BW) on serum SOD levels in normal, untreated and treated diabetic rats to determine whether AO can increase the endogenous antioxidant pool. Our results showed that AO (100 mg/kg BW) increased serum SOD level significantly (p < 0.05) when compared to diabetic rats that have not been treated with either AO or glibenclamide (Fig. 8b).

4. DISCUSSION
4.1. Body Weight, Glucose Level and Glucose Tolerance
The current study showed that AO plant extract had no effect on body weight gain in diabetic rats. Since this is the first study that examined the effect of AO on body weight gain in rats, it is difficult to assign a reference point. In addition to the data on the effect of AO on body weight, the present study showed that at the end of the study, there was no obvious lowering effect of fasting blood glucose after AO treatment in diabetic rats down to the normal value. However, on day 10 of the treatment, there was a significant reduction in the level of fasting blood glucose in diabetic rats treated with AO (100 mg/kg BW) compared to untreated diabetic rats, but this effect did not persist to the end of the study. The reason for this effect is unknown. It may be due to the quantity of the dose and/or duration of the study. In addition, the intraperitoneal glucose tolerance test (IPGTT) showed that glucose handling was not improved in diabetic rats after treatment with AO. Other herbal extract, such as Momordica charantia, can lower the blood glucose level when administered to diabetic rats [28].
4.2. Morphological and Immunohistochemical Study
The results obtained from this study confirmed that the light microscopic structures of the pancreas were near normal, 30 days after treatment with different doses of AO. The overall picture of the protective effects of AO treatment in diabetic rats was evidenced with an improved H & E staining of histological structures in both liver and kidney, which had near normal morphology. The overall effect of AO treatment or its derivative, morin, has been reported to be associated mainly with improving the signs of many diseases such as heart disease, neuropathy, cancer, inflammatory disorders, impaired oxidative stress, liver toxicity, and kidney complaints [29].
There is always an abnormal distribution pattern of both insulin- and glucagon-positive cells within the pancreatic islets of Langerhans in diabetic rats. This abnormal pattern is associated with reduced insulin secreting beta cells and a concomitant increase in the number of glucagon secreting alpha cells [30, 31]. The number of these beta and alpha cells improved after treatment with AO 200 mg/kg BW. These findings revealed the protective effect of AO extract in increasing the number of beta cells with a concomitant reduction in the number of glucagon-secreting cells. This observation may be due to the increasing neogenesis of beta cells. The ability of medicinal plants in the initiation of neogenesis has already been reported [28, 32]. The increase in the number of pancreatic beta cells may also be due to a reduction in cell apoptosis. In fact, previous studies [33] showed that active ingredients of AO have anti-apoptotic activity. We did not observe significant increases in the serum insulin level in diabetic rats treated with AO, albeit increases in the number of insulin-positive cells were observed. It is possible that the increase in the number of insulin-producing cells in the islets of Langerhans was not significant to increase the blood concentration of insulin.
However, our study showed a significant reduction in the number of glucagon-positive cells in AO-treated diabetic rats. Since insulin inhibits glucagon, an increased level of insulin will always be associated with a decrease in glucagon concentration. All of these indicate a protective nature of AO on STZ-induced diabetes since glucagon is always up-regulated after the onset of diabetes [34].
4.3. Liver and Kidney Function Tests
The current study also showed the beneficial effect of AO on liver functions. AST and ALT liver enzymes as markers of liver function demonstrated no significant variations between all study groups. These results mean that AO is safe and nontoxic to diabetic rat liver. Serum bilirubin was significantly reduced in diabetic rats after treatment with AO (100 and 200 mg/kg BW), and glibenclamide. These results revealed that AO has a putative beneficial hepatoprotective effect on diabetic rats. This observation is consistent with the reported antioxidant and anti-lipoxygenase properties of AO [35].
Our study showed that AO treatment has a protective effect on kidney functions after the onset of diabetes, as evidenced by a reduction in the serum level of kidney function markers such as serum creatinine. However, AO failed to alter the BUN level in diabetic rats. These results showed that AO 200 mg/kg BW has a beneficial effect on the amelioration of the complications of DM. The ability of AO to protect the kidney and improve on kidney function markers may lie in the fact that AO contains morins, a potent component of flavonoids. Morins have a potent free radical scavenging and anti-lipid peroxidation activities [36, 37]. The fact that AO did not reduce the blood level of BUN may be due to the dosage regimen and the duration of the study.
4.4. Serum Insulin and SOD
Our study clearly showed that AO can increase the blood concentration of SOD, a potent endogenous antioxidant. The beneficial action of AO may be related to its bioactive flavonoid compound morin, which was isolated from aerial samples of AO [14]. Morin is a powerful active compound, which has many functions such as antioxidant, anti-inflammatory, neuro-protective, and anti-apoptotic actions [38, 39]. The ability of AO to increase the antioxidant pool in diabetic rat serum may also be due to the polyphenolic compounds of AO [40].
The effect of AO exhibited in this study may be due to its morin content. Morin, a flavonoid within the larger class of polyphenols, was extracted from AO [14]. In addition, reports have shown that morin is present in osage orange and the leaves of common guava [41]. In previously reported studies, morin inhibited fatty acid synthase, the enzyme responsible for the formation of tissue fat [42]. It also prevents the deposition of amylin in pancreatic islets [43]. Amylin is released with insulin in pancreatic islets, where excessive deposition leads to the development of DM [44]. Other antioxidants, such as tannins, have also been isolated from AO [45]. All of these antioxidants, including morin, may play a role in the mitigation of the signs of DM
CONCLUSION
In summary, the present study showed the beneficial effects of AO on diabetic rats after 30 days of treatment. AO extract has a beneficial impact on liver and kidney structure and function. In addition, AO increased the antioxidant pool and the number of insulin-secreting beta cells with a concomitant decrease in the number of glucagon-producing cells. All of these observations point to the potential role of AO in the amelioration of the signs of diabetes mellitus. Since this is the first study on the potential effect of AO on diabetes mellitus, more studies are needed to explore additional potentials of this medicinal plant.
LIMITATION OF THE STUDY
The effect of AO on the morphology of the pancreas, liver, and kidney and on hepatic, renal and pancreatic functions was carried out on streptozotocin-induced diabetes. This model of DM is similar to type 1 DM rather than type 2 DM. Streptozotocin causes severe destruction of pancreatic beta cells [46], a type that may not completely resemble those seen in human models of DM. Moreover, a higher dose regimen may have a more profound benefit in this animal model.
LIST OF ABBREVIATIONS
| DM | = Diabetes Mellitus. |
| AO | = Acridocarpus orientalis. |
| BW | = Body Weight. |
| IDF | = International Diabetes Federation. |
| SOD | = Superoxide Dismutase. |
| O2.- | = Superoxide Anion. |
| IPGTT | = Intraperitoneal Glucose Tolerance Test. |
| H & E | = Haematoxylin and Eosin. |
| AST | = Aspartate Aminotransferase. |
| ALT | = Alanine Aminotransferase. |
AUTHOR'S CONTRIBUTIONS
ML, TK, EA, ARP, CD planned the experiment, analyzed data and wrote the manuscript, ML, ARP and CD performed the experiments and ML, SM, EA analyzed data and finalized the manuscript. All the authors approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by ethic committee of the College of Medicine & Health Sciences, United Arab Emirates University, UAE, (Ethical clearance number: A2-R07).
HUMAN AND ANIMAL RIGHTS
No humans were used. The reported experiments on animals were in accordance of Animal Research Group guidelines for the care and use of laboratory animals (College of Medicine & Health Sciences, United Arab Emirates University, UAE).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The project was funded by UAEU UPAR Grant # 31S163), and research grants from the College of Medicine & Health Sciences (NP-15-41) UAE.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the Department of Biology, College of Science, Department of Anatomy, College of Medicine & Health Sciences, and the UAE University for creating an environment that encourages research.


