All published articles of this journal are available on ScienceDirect.
Antiviral Activity of Benzotriazole Based Derivatives
Abstract
Background:
For the last thirty years, the benzotriazole scaffold has been the object of our group interest and we have already presented some results on the antiviral activity of our compounds.
Objective:
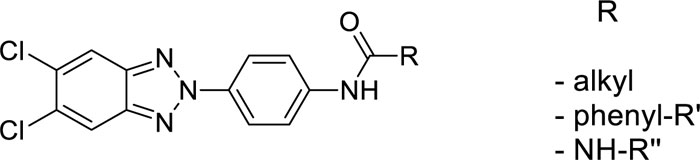
In this article, we conclude the exploration of N-(4-(R-2H-benzo[d][1,2,3]triazol-2-yl)phenyl)-4-R’-benzamides and 1-(4-(R-2H-benzo[d][1,2,3]triazol-2-yl)phenyl)-3-R’-ureas by synthesizing further modified derivatives, in order to have more elements for SARs evaluation.
Methods:
Here, we reported the synthesis and the antiviral screening results of 38 newly synthesized benzotriazole derivatives against a panel of DNA and RNA viruses. We also analyse SARs in comparing these compounds with previously published benzotriazole analogues, taking stock of the situation.
Results:
Among the newly presented derivatives, compounds 17 and 18 were the most active with EC50 6.9 and 5.5 µM, respectively against Coxsackievirus B5 (CV-B5) and 20.5 and 17.5 µM against Poliovirus (Sb-1).
Conclusion:
we can conclude that N-(4-(2H-benzo[d] [1 - 3] triazol-2-yl)phenyl-R-amide is a good chemical scaffold for the development of new antiviral molecules.
1. INTRODUCTION
Benzotriazole and its derivatives have a great importance in the biomedical research. The chemical activities of this scaffold are manifold; it can be used as intermediate [1-3], leaving group [4], or inserted into other chemical structures [5]. The antimicrobial activity of benzotriazole derivatives has been extensively investigated since the late 1980s and several authors disclosed the biological evaluation of imidazole derivatives and benzotriazole analogues as antibacterial, antimycotic, antitubercular, and antiviral agents [6]. The number of viruses and the viral infections are continuously increasing. Poverty, rapid urbanization, evolving human migration patterns, re-emerging viruses, all contribute to the expanding impact of viral diseases. Environmental variations and derangements caused by changes in the climate and alterations to the ecology caused by man have increased the possibility for new contacts between the various factors involved, giving rise conditions for the emergence of new infections. This is reflected by the recent emergence of viral outbreaks caused by West Nile virus (WNV), severe acute respiratory syndrome (SARS) virus, Enterovirus A71 and D68, influenza virus, measles virus (MV) [7-9] and coronavirus (SARS-CoV-2) nowadays [10]. While vaccine efforts have been proven successful for preventing and eradicating some viral infections, for many viruses there is not any vaccine, efficacious drugs for prophylaxis or therapeutic treatments available. Thus, more attempts in developing antiviral therapies are needed to protect public health against a range of important viral pathogens. Progress has been made recently on azole derivatives as antibacterial, antifungal, antitubercular and antiviral agents, including mono-nitrogen azoles (oxazoles, thiazoles and carbazoles), bis-nitrogen azoles (imidazoles, pyrazoles and benzimidazoles) and tri-nitrogen azoles (triazoles and benzotriazoles) [11-14]. Starting from these considerations and among a multi-annual antiviral research program, in the last years, we described the chemical characterization and antiviral activity of several series of azole derivatives as benzimidazole, benzotriazoles [15-17], and a large series of both benzimidazole and benzotriazole derivatives condensed with a pyridine ring [18, 19]. Among these azoles, benzotriazoles derivatives bearing one or two chlorine atoms on the benzene moiety of benzotriazole, in positions 5 and 6, have proved to be of particular interest. From an extensive screening against both RNA and DNA viruses, these derivates were found to be active against BVDV and hRSV [16], while the elimination of a chlorine atom on the same scaffold resulted in the loss of activity on all viral strains tested [16].
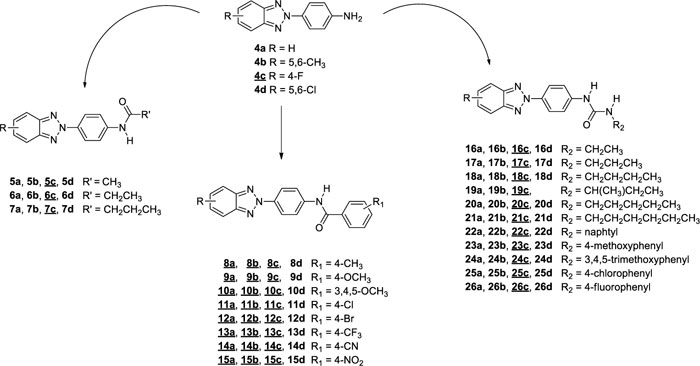
This project aimed to evaluate how changes on the benzotriazole scaffold, or in the amide portion, could affect the antiviral activity of previously reported derivatives. Starting from compounds with general structure depicted in Fig. (1)., which turned out active towards Coxsackieviruses, BVDV and hRSV [16], we designed and synthesised a series of derivatives 4-7c, 8a,b,c-15a,b,c, 16c-26c (underline-labelled in Fig. (2). and compared their antiviral activity with previously published compounds (4a,b-26a,b, 16d-18d, 20d-26d; not underline-labelled in Fig. (2). describing Structure-activity Relationships (SARs).
In all these derivatives, we evaluated the role of the substitution of the benzotriazole ring with hydrogen, fluorine, chlorine atoms, or methyl groups. Similarly, the replacement of the amide moiety with an ureidic function on all series of new benzotriazole derivatives was assessed, since previously synthesised similar compounds (16a,b-26a,b, 16d-18d, 20d-26d) had shown interesting antiviral activity [16, 17], modification of the chemical scaffold are outlined in Fig. (3).

All newly prepared derivatives were assayed against representatives positive- and negative- sense single-stranded RNA, double-stranded RNA viruses and DNA viruses. In order to establish whether tested compounds worked selectively as antiviral agents, their cytotoxicity against cell monolayers (in stationary growth) analogous to those supporting the replication of the above viruses, but left uninfected, was evaluated in parallel with their antiviral activity.
2. MATERIALS AND METHODS
2.1. Synthetic Methods
Melting points (m.p.) were carried out with a Köfler hot stage or Digital Electrothermal melting point apparatus. Nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were determined in CDCl3 or DMSO-d6 and recorded with a Bruker Avance III 400 NanoBay. Chemical shifts are reported in parts per million (ppm) downfield from tetramethylsilane (TMS) used as internal standard. Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; quin, quintet; sext, sextet; sept, septet; m, multiplet; br s, broad singlet; dd, double doublet. Mass spectra (MS) were performed on the combined Liquid Chromatograph-Agilent 1100 series Mass Selective Detector (MSD). Analytical thin-layer Chromatography (TLC) was performed on Merck silica gel F-254 plates. Pure compounds showed a single spot in TLC. For flash chromatography, Merck silica gel 60 was used with a particle size of 0.040-0.063mm (230-400 mesh ASTM). Elemental analyses were performed on a Perkin-Elmer 2400 instrument and the results were within ±0.4% of theoretical values.
2.2. Starting Material and Known Intermediates
Anhydrides, benzoyl derivatives, 1-chloro-4-nitrobenzene, and inorganic reagent were commercially available. The intermediate 3-fluorobenzene-1,2-diamine, common to the whole series, was prepared according to the procedures described in the literature [20, 21] . Details of the synthesis of each compound are provided as follows.
2.3. Cells and Viruses
Cell lines were purchased from American Type Culture Collection (ATCC). Cell lines supporting the multiplication of virus were following: Monkey kidney (Vero-76) [ATCC CRL 1587 Cercopithecus Aethiops]. Viruses were purchased from the American Type Culture Collection (ATCC). Viruses representative of positive-sense, single-stranded RNAs (ssRNA+) were: Picornaviridae: human enterovirus B [coxsackie type B5 (CV-B5), strain Faulkner (ATCC VR-185)], and human enterovirus C [poliovirus type-1 (Sb-1), Sabin strain Chat (ATCC VR-1562)]. Cell cultures were checked periodically for the absence of mycoplasma contamination with MycoTect Kit (Gibco). Viruses were maintained in our laboratory and propagated in appropriate cell lines. The viruses were stored in small aliquots at -80 °C until use.
2.4. Cytotoxicity Assays
Vero-76 cells were seeded in 96-well plates at an initial density of 5x105 cells/mL, in Dulbecco’s Modified Eagle Medium (D-MEM) with L-glutamine and 25 mg/L kanamycin, supplemented with 10% FBS. Cell cultures were then incubated at 37 °C in a humidified, 5% CO2 atmosphere, in the absence or presence of serial dilutions of test compounds. The test medium used for the cytotoxic assay as well as for antiviral assay contained 1% of the appropriate serum. Cell viability was determined after 72 hrs at 37 °C by the MTT method [22].
2.5. Antiviral Assay
Compound’s activity against CV-B5 and Sb-1 was determined by plaque reduction assays in infected cell monolayers. Briefly, a monolayer of Vero-76 cells was grown overnight on 24-well plate. The cells were then infected for 2 hrs with 250 μL of proper virus dilutions to give 50-100 PFU/well. Following the removal of unadsorbed virus, 500 μL of the medium [D-MEM with L-glutamine and 4500 mg/L D-glucose, supplemented with 1% inactivated FBS] containing 0.75% methylcellulose, with serial dilutions of test compounds, were added. The overlayed medium was also added to not treated wells as non-infection controls. Cultures were incubated at 37 °C for 2 (Sb-1), 3 (CV-B5) days and then fixed with PBS containing 50% ethanol and 0.8% crystal violet, washed and air-dried. Plaques in the control (no inhibitor) and experimental wells were then counted.
2.6. Linear Regression Analysis
The extent of cell growth/viability and viral multiplication, at each drug concentration tested, were expressed as a percentage of untreated controls. Concentrations resulting in 50% inhibition (CC50 or EC50) were determined by linear regression analysis.
2.7. Experimental Section
2.7.1. General procedure for the preparation of 7-fluoro-1-(4-nitrophenyl)-1H-benzo[d] [1-3]triazole (1), 4-fluoro-2-(4-nitrophenyl)-2H-benzo[d] [1-3]triazole (2) and 4-fluoro-1-(4-nitrophenyl)-1H-benzo[d] [1-3]triazole (3).
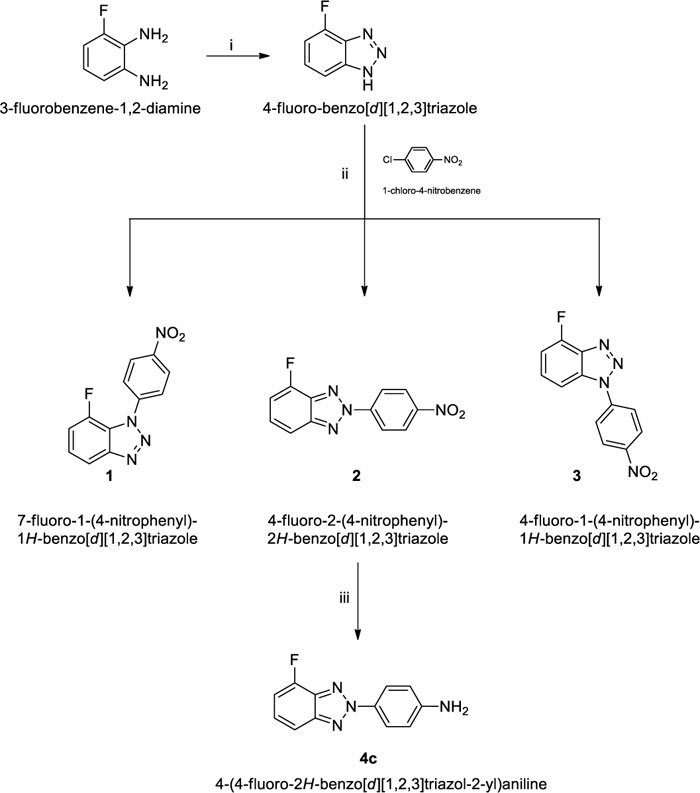
To a solution of 2 g (14.6 mmol) of 4-fluoro-1H-benzo[d] [1-3] triazole in 14 mL of dimethyl acetamide (DMA), 1-chloro-4-nitrobenzene (2.3 g, 14.6 mmol) and Cs2CO3 (9.51 g, 29.2 mmol) were added. The mixture was stirred at 75°C for 36 h. The precipitate was filtered by washing a little amount of water to remove Cs2CO3, and the filtrate was poured on ice. The obtained light-yellow precipitate was the mixture of the three isomers and was filtered under vacuum. The three isomers were separated by flash chromatography using petroleum ether/ethyl acetate in different ratio as eluting system. The assignment of the correct structure to the isomers (1), (2) and (3) was achieved from NOESY experiment as discussed in paragraph 2.1 of this article.
2.7.1.1. 7-fluoro-1-(4-nitrophenyl)-1H-benzo[d] [1-3]triazole (1)
Compound (1) was purified by silica gel column chromatography eluting with a 9:1 mixture of petroleum ether/ethyl acetate. Light-yellow solid, m.p.: 188-191°C.Yield, 1%. TLC (petroleum ether/ethyl acetate 9:1): Rf 0.22. 1H-NMR (DMSO, 400 MHz) δ: 8.51 (2H, d, J = 8.4 Hz, H-3’,5’), 8.13 (2H, d, J = 8.4 Hz, H-2’,6’), 8.091 (1H, d, J = 8 Hz, H-4), 7.59-7.55 (2H, m, H-5,6). 13C-NMR (CDCl3, 100 MHz) δ: 149.5 (C, C-7), 148.4 (C, C-3a), 147.3 (C, C-7a), 145.7 (C, C-4’), 140.9 (C, C-1’), 126.0 (CH, C-5), 125.49 (CH, C-2’, C-6’), 124.91 (CH, C-3’, 5’), 116.20 (CH, C-4), 114.23 (CH, C-6). LC/MS: m/z 259 (M+H). Anal. Calcd. (%) C12H7FN4O2: C, 55.82; H, 2.73; N, 21.70. Found: C, 55.94; H, 2.77; N, 21.84.
2.7.1.2. 4-fluoro-2-(4-nitrophenyl)-2H-benzo[d] [1-3]triazole (2)
Compound (2) was purified by silica gel column chromatography eluting with a 9.8:0.2 mixture of petroleum ether/ethyl acetate. Light-yellow solid, m.p.:234-237°C. Yield, 37%. TLC (petroleum ether/ethyl acetate 9:1): Rf 0.46. 1H-NMR (DMSO, 400 MHz) δ: 8.59 (2H, d, J = 9.2 Hz, H-2̍,6̍), 8.51 (2H, d, J = 9.2 Hz, H-3̍,5̍), 7.93 (1H, d, J = 8.8 Hz, H-7), 7.60-7.54 (1H, m, H-6), 7.41 (1H, dd, J = 7.2 and 10.8 Hz, H-5). 13C-NMR (DMSO, 100 MHz) δ: 152.8 (C, C-4). 147.5 (C, C-7a), 147.3 (C, C-1’), 136.2 (C, C-3a), 134.1 (C, C-4’), 125.6 (CH, C-6), 124.4 (CH, C-7), 121.5 (CH, C-2’, C-6’), 115.1 (CH, C-3’, 5’), 111.6 (CH, C-5). LC/MS: m/z 259 (M+H). Anal. Calcd. (%) C12H7FN4O2: C, 55.82; H, 2.73; N, 21.70. Found: C, 55.92; H, 2.78; N, 21.85.
2.7.1.3. 4-fluoro-1-(4-nitrophenyl)-1H-benzo[d] [1-3]triazole (3)
Compound (3) was purified by silica gel column chromatography eluting with a 8.5:1.5 mixture of petroleum ether/ethyl acetate. Light-yellow solid, m.p.: 233-236°C. Yield, 2%. TLC (petroleum ether/ethyl acetate 9:1): Rf 0.16. 1H-NMR (DMSO, 400 MHz) δ: 8.52 (2H, d, J = 8.8 Hz, H-3̍, 5̍), 8.25 (2H, d, J = 8.8 Hz, H-2̍, 6̍), 7.92 (1H, d, J = 8.4 Hz, H-7), 7,61 (1H, m, H-6), 7,44 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 152.4 (C, C-4), 146.9 (C, C-7a), 140.8 (C, C-1’), 136.0 (C, C-3a), 134.4 (C, C-4’), 125.6 (CH, C-6), 123.4 (CH, C-2’, C-6’), 110.0 (CH, C-7), 109.9 (CH, C-3’, 5’), 107.9 (CH, C-5,). LC/MS: m/z 259 (M+H). Anal. Calcd. (%) C12H7FN4O2: C, 55.82; H, 2.73; N, 21.70. Found: C, 55.90; H, 2.80; N, 21.83.
2.7.2. 4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)aniline (4c)
Compound (4c) was obtained by dissolving 2g of derivative 2 (7.74 mmol) in 269 mL of ethanol followed by the addition of 10% Palladium on activated charcoal and hydrazine hydrate (3.47 g, 108.08 mmol). The mixture was heated at 110°C for 20 h, after cooling the catalyst was filtered and washed with ethanol, the filtrate was evaporated under reduced pressure. The crude product was purified by flash chromatography using petroleum ether/ethyl acetate 7:3 as eluting system. 933 mg of 4c was obtained as a light-yellow solid, m.p. 167-169°C (from EtOH). Yield: 53%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.41. 1H-NMR (CDCl3, 400 MHz) δ: 8.05 (2H, d, J= 7.6 Hz, H-2̍, 6̍), 7.62 (1H, d, J= 8.4 Hz, H-7), 7.26-7.21 (1H, m, H-6), 6.96 (1H, t, H-5), 6.73 (2H, d, J= 7.6 Hz, H-3̍, 5̍), 3.89 (2H, br s, NH2). 13C-NMR (CDCl3, 100 MHz) δ: 152.5 (C, C-4), 147.8 (C, C-7a), 147.7 (C, C-1’), 135.9 (C, C-3a), 131.8 (C, C-4’), 126.5 (CH, C-6), 122.2 (CH, C-3’, C-5’), 115.0 (CH, C-2’, C-6’), 114.0 (CH, C-7), 109.8 (CH, C-5). LC/MS: m/z 229 (M+H). Anal. Calcd. (%) C12H9FN4: C, 63.15; H, 3.97; N, 24.55. Found: C, 63.24; H, 4.06; N, 24.65.
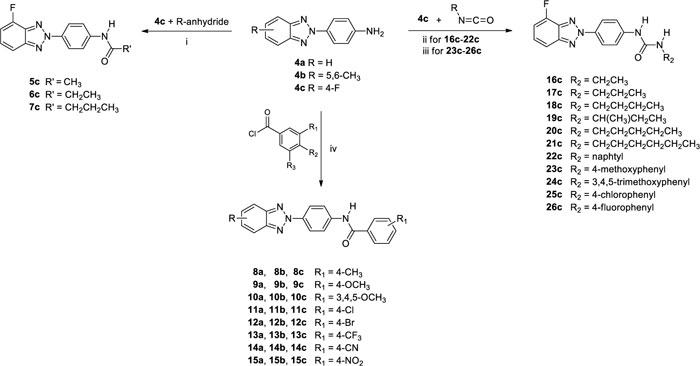
2.7.3. General procedure for the preparation of N-(4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)phenyl)amide derivatives (5c-7c).
To 4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)aniline (4c) (200 mg, 0.87 mmol) was added an excess (26.1 mmol) of either required anhydride (acetic anhydride, propionic anhydride and butyric anhydride). The resulting mixture was stirred at 100°C for different times as reported below, then it was cooled to room temperature and crushed ice was added. The solid obtained in each reaction was filtered under vacuum. Purification by flash chromatography using as eluting system, a mixture of petroleum ether/ethyl acetate in different ratios as reported below, furnished the amide derivatives 5c-7c.
2.7.3.1. 1N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl) phenyl) acetamide (5c)
Compound (5c) was obtained as described in the general procedure starting from 4c and acetic anhydride for 6h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 191-194°C (from EtOH). Yield, 28%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.45. 1H-NMR (CDCl3, 400 MHz) δ: 10.13 (1H, br s, NH), 8.52 (2H, d, J= 8.4 Hz, H-3̍,5̍), 7.65 (1H, d, J= 8.8 Hz, H-7), 7.38 (2H, d, J= 8.8 Hz, H-2̍,6̍), 7.40-7.27 (1H, m, H-6), 7.09 (1H, t, H-5), 2.35 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 172.6 (C, CO), 152.6 (C, C-4), 147.6 (C, C-7a), 140.2 (C, C-1’), 140.1 (C, C-4’), 136.7 (C, C-3a), 131.0 (CH, C-3’, C-5’), 127. (CH, C-6), 122.0 (CH, C-2’, C-6’), 114.6 (CH, C-7), 110.7 (CH, C-5), 25.8 (CH3). LC/MS: m/z 271 (M+H). Anal. Calcd. (%) C14H11FN4O: C, 62.22; H, 4.10; N, 20.73. Found: C, 62.33; H, 4.15; N, 20.80.
2.7.3.2. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl) phenyl) propionamide (6c)
Compound (6c) was obtained as described in the general procedure starting from 4c and propionic anhydride for 72h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Ocra yellow solid, m.p.: 179-182°C (from EtOH). Yield, 26%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.51. 1H-NMR (CDCl3, 400 MHz) δ: 8.319 (2H, d, J= 8.8 Hz, H-3̍,5̍), 7.76 (1H, d, J= 8.4 Hz, H-7), 7.70 (2H, d, J= 8.8 Hz, H-2̍,6̍), 7.48 (1H, br s, NH), 7.36-7.26 (1H, m, H-6), 7.07-7.05 (1H, t, H-5), 2.45 (2H, d, CH2), 1.28 (3H, t, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 172.2 (C, CO), 152.5 (C, C-4), 147.4 (C, C-7a), 139.1 (C, C-1’), 136.2 (C, C-4’), 135.9 (C, C-3a), 127.0 (CH, C-6), 121.4 (CH, C-3’, C-5’), 118.2 (CH, C-2’, C-6’), 114.3 (CH, C-7), 110.2 (CH, C-5), 30.8 (CH2, CH2CH3), 9.55 (CH3, CH2CH3). LC/MS: m/z 285 (M+H). Anal. Calcd. (%) C15H13FN4O: C, 63.37; H, 4.61; N, 19.71. Found: C, 64.07; H, 4.69; N, 19.75.
2.7.3.3. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl) phenyl) butyramide (7c)
Compound (7c) was obtained as described in the general procedure starting from 4c and butyric anhydride for 120h. The crude product was purified by silica gel column chromatography eluting with a 9:1 mixture of petroleum ether/ethyl acetate. Wight solid, m.p.: 208-209°C (from EtOH). Yield, 31%. TLC (petroleum ether/ethyl acetate 8:2): Rf 0.48. 1H-NMR (DMSO, 400 MHz) δ: 10.25 (1H, br s, NH), 8.26 (2H, d, J = 8.8 Hz, H-3̍,5̍), 7.89 (2H, d, J = 8.8 Hz, H-2̍,6̍), 7.88 (1H, d, J = 8.0 Hz, H-7), 7.52-7.47 (1H, m, H-6), 7.35-7.30 (1H, m, H-5), 2.35 (2H, t, CH2), 1.65 (2H, sext., CH2), 0.94 (3H, t, CH3). 13C-NMR (DMSO, 100 MHz) δ: 171.8 (C, CO), 151.4 (C, C-4), 146.8 (C, C-7a), 140.5 (C, C-1’), 135.2 (C, C-3a), 134.2 (C, C-4’), 127.6 (CH, C-6), 120.9 (CH, C-3’, C-5’), 119.7 (CH, C-2’, C-6’), 112.6 (CH, C-7), 110.7 (CH, C-5), 18.4 (CH2, CH2-CH2CH3), 13.5 (CH3, CH2-CH2-CH3). LC/MS: m/z 299 (M+H). Anal. Calcd. (%) C16H15FN4O: C, 64.42; H, 5.07; N, 18.78. Found: C, 64.51; H, 4.75; N, 19.82.
2.7.4. General procedure for the preparation of N-(4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)phenyl)benzamide derivatives (8a,b,c-15a,b,c).
The respective amines 4a-c (166 mmol) were solubilized in 8-10 ml of DMF (compounds a,b) or DMA (compounds c) and an equimolar amount (compounds c) or an equimolar amount with 20% in excess (compounds a,b) of the required benzoyl chloride derivatives was added. The solution was stirred at 80°C for different times as reported below, then it was cooled to room temperature and crushed ice was added. The reaction products were solid or a gummy oil; The solid obtained was filtered under vacuum, while the gummy oil was treated with ether. Purification by flash chromatography using as eluting system, a mixture of petroleum ether/ethyl acetate in different ratios as reported below, furnished the benzamide derivatives 8a,b,c-15a,b,c.
2.7.4.1. N-(4-(2H-benzo[d] [1-3] triazol-2-yl) phenyl) -4-methylbenzamide (8a)
Compound (8a) was obtained as described in the general procedure starting from 4a and 4-methylbenzoyl chloride for 24h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 266-268°C (from EtOH). Yield, 80%. TLC (petroleum ether/ethyl acetate 1:1): Rf 0.90. 1H-NMR (DMSO, 400 MHz) δ: 10.49 (1H, s, NHCO), 8.32 (2H, d, J=8.4 Hz, H-2’, 6’), 8.09 (2H, d, J= 8.4 Hz, H-3’, 5’), 8.05-8.00 (2H, m, H-4, 7), 7.92 (2H, d, J= 7.6 Hz, H-2”, 6”), 7.53-7.50 (2H, m, H-5, 6), 7.37 (2H, d, J= 7.6 Hz, H-3”, 5”). 13C-NMR (DMSO, 100 MHz) δ: 165.6 (C, CO), 144.3 (C, C-4”), 141.9 (C, C-3a, C-7a), 140.4 (C, C-4’), 134.9 (C, C-1’), 131.7 (C, C-1”), 128.9 (CH, C-3”, C-5”), 127.8 (CH, C-2”, C-6”), 127.4 (CH, C-5, C-6), 120.9 (CH, C-2’, C-6’), 120.7 (CH, C-3’, C-5’), 118.0 (CH, C-4, C-7), 21.0 (CH3). LC/MS: m/z: 329 (M+H). Anal. Calcd (%) C20H16N4O: C, 73.15; H; 4.91; N, 17.06. Found: C, 73.41; H, 4.98; N, 16.91.
2.7.4.2. N-(4-(2H-benzo[d] [1-3]triazol-2-yl)phenyl)-4-methoxy benzamide (9a)
Compound (9a) was obtained as described in the general procedure starting from 4a and 4-methoxybenzoyl chloride for 48h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 217-218°C (from EtOH). Yield, 90%. TLC (petroleum ether/ethyl acetate 1:1): Rf 0.81. 1H-NMR (DMSO, 400MHz) δ: 10.42 (1H, s, NHCO), 8.31 (2H, d, J= 8.4 Hz, H-2”, 6”), 8.08 (2H, d, J= 8.0 Hz, H-3”, 5”), 8.18-7.98 (4H, m, H-2’, 6’, 3’, 5’), 7.53-7.49 (2H, m, H-4, 7), 7.15-7.03 (2H, m, H-5, 6). 13C-NMR (DMSO, 100 MHz) δ: 165.1 (C, CO), 162.1 (C, O-C), 144.3 (C, C-3a, C-7a), 140.5 (C, C-4’), 134.8 (C, C-1’), 129.7 (CH, C-2”, C-6”), 127.4 (CH, C-5, C-6), 126.6 (C, C-1”), 120.8 (CH, C-2’, C-6’), 120.7 (CH, C-3’, C-5’), 118.1 (CH, C-4, C-7), 113.7 (CH, C-3”, C-5”), 55.4 (OCH3). LC/MS: m/z: 345 (M+H). Anal. Calcd (%) C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.41; H, 4.43; N, 16.50.
2.7.4.3. N-(4-(2H-benzo[d] [1-3] triazol-2-yl) phenyl)-3,4,5-trimethoxybenzamide (10a)
Compound (10a) was obtained as described in the general procedure starting from 4a and 3,4,5-trimethoxybenzoyl chloride for 2 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 217-229°C (from EtOH). Yield, 30%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.29. 1H NMR (DMSO, 400 MHz) δ: 10.44 (1H, s, NHCO), 8.34 (2H, d, J=8.4 Hz, H-2’, 6’), 8.04-8.00 (4H, m, H-4, 7, 3’, 5’), 7.53-7.50 (2H, m, H-5, 6), 7.33-7.28 (2H, m, H-2”, 6”). 13C-NMR (DMSO, 100 MHz) δ: 165.2 (C, CO), 152.6 (C, C-3”, C-5”), 144.4 (C, C-3a, C-7a), 140.4 (C, C-4”), 135.0 (C, C-1”), 129.7 (C, C-1’, C-4’), 127.5 (CH, C-5, C-6), 121.2 (CH, C-2’, C-6’), 120.7 (CH, C-3’, C-5’), 118.0 (CH, C-4, C-7), 105.4 (CH, C-2”, C-6”), 60.1 (OCH3-C4”), 56.1 (OCH3-C3”, OCH3-C5”). LC/MS: m/z: 405 (M+H). Anal. Calcd (%) C22H20N4O4: C, 65.34; H, 4.98; N, 13.85. Found: C, 64.98; H, 4.63; N, 14.20.
2.7.4.4. N-(4-(2H-benzo[d] [1-3] triazol-2-yl) phenyl) -4-chlorobenzamide (11a)
Compound (11a) was obtained as described in the general procedure starting from 4a and 4-chlorobenzoyl chloride for 2 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 280-281°C (from EtOH). Yield, 68%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.83. 1H-NMR (DMSO, 400 MHz) δ: 10.64 (1H, s, NHCO), 8.33 (2H, d, J= 8.4 Hz, H-2’, 6’), 8.08 (2H, d, J= 8.8 Hz, H-2”, 6”), 8.08-8.00 (4H, m, H-3’, 5’, 3”, 5”), 7.69-7.63 (2H, m, H-4, 7), 7.57-7.50 (2H, m, H-5, 6). 13C-NMR (DMSO, 100 MHz) δ: 164.7 (C, CO), 144.4 (C, C-3a, C-7a), 140.0 (C, C-4”), 136.6 (C, C-4’), 135.1 (C, C-1’), 133.3 (C, C-1”), 129.7 (CH, C-2”, C-6”), 128.5 (CH, C-3”, C-5”), 127.5 (CH, C-5, C-6), 121.0 (CH, C-2’, C-6’), 120.7 (CH, C-3’, C-5’), 118.0 (CH, C-4, C-7). LC/MS: m/z: 349 (M+H). Anal. Calcd (%) C19H13ClN4O: C, 65.43; H, 3.76; N, 16.06. Found: C, 65.66; H, 4.04; N, 16.00.
2.7.4.5. N-(4-(2H-benzo[d] [1-3] triazol-2-yl) phenyl) -4-bromobenzamide (12a)
Compound (12a) was obtained as described in the general procedure starting from 4a and 4-bromobenzoyl chloride for 2 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 248-249°C (from EtOH). Yield, 79%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.72. 1H-NMR (DMSO, 400 MHz) δ: 10.64 (1H, s, NHCO), 8.33 (2H, d, J= 8.4 Hz, H-2’, 6’), 8.07 (2H, d, J= 8.4 Hz, H-3’, 5’), 8.03-8.01 (2H, m, H-4, 7), 7.96 (2H, d, J= 8.0 Hz, H-2”, 6”), 7.79 (2H, d, J= 8.4 Hz, H-3”, 5”), 7.53-7.49 (2H, m, H-5, 6). 13C-NMR (DMSO, 100 MHz) δ: 164.8 (C, CO), 144.4 (C, C-3a, C-7a), 140.0 (C, C-4’), 135.1 (C, C-1’), 133.7 (C, C-1”), 131.5 (CH, C-3”, C-5”), 129.9 (CH, C-2”, C-6”), 127.5 (CH, C-5, C-6), 125.6 (C, C-4”), 121.0 (CH, C-2’, C-6’), 120.8 (CH, C-3’, C-5’), 118.1 (CH, C-4, C-7). LC/MS: m/z: 395 (M+H). Anal. Calcd (%) C19H13BrN4O: C, 58.03; H, 3.33; N, 14.25. Found: C, 58.23; H, 3.54; N, 14.00.
2.7.4.6. N-(4-(2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-(trifluoromethyl)benzamide (13a)
Compound (13a) was obtained as described in the general procedure starting from 4a and 4-trifluorobenzoyl chloride for 2 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 277-278°C (from EtOH). Yield, 72%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.79. 1H-NMR (DMSO, 400 MHz) δ: 10.79 (1H, s, NHCO), 8.35 (2H, d, J= 8.8 Hz, H-2’, 6’), 8.20 (2H, d, J= 7.6 Hz, H-2”, 6”), 8.09 (2H, d, J= 8.4 Hz, H-3’, 5’), 8.03-8.00 (2H, m, H-4, 7), 7.96 (2H, d, J= 8.0 Hz, H-3”, 5”), 7.54-7.50 (2H, m, H-5, 6). 13C-NMR (DMSO, 100 MHz) δ: 164.6 (C, CO), 144.4 (C, C-3a, C-7a), 139.9 (C, C-4’, C-1”), 138.4 (C, C-1’), 135.3 (C, C-4”), 131.5 (C, CF3), 128.7 (CH, C-2”, C-6”), 127.5 (CH, C-5, C-6), 125.4 (CH, C-3”, C-5”), 121.1 (CH, C-2’, C-6’), 120.8 (CH, C-3’, C-5’), 118.1 (CH, C-4, C-7). LC/MS: m/z: 383 (M+H). Anal. Calcd (%) C20H13F3N4O: C, 62.83; H, 3.43; N, 14.65. Found: C, 62.52; H, 3.23; N, 14.86.
2.7.4.7. N-(4-(2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-cyanobenzamide (14a)
Compound (14a) was obtained as described in the general procedure starting from 4a and 4-cyanobenzoyl chloride for 48h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 243-245°C (from EtOH). Yield, 22%. TLC (petroleum ether/ethyl acetate 75:25): Rf 0.13. 1H-NMR (DMSO, 400 MHz) δ: 10.59 (1H, s, NHCO), 8.40 (2H, d, J= 13.6 Hz, H-4, 7), 8.34 (2H, d, J= 8.4 Hz, H-2’, 6’), 8.14-8.06 (3H, m, H-5, 2”, 6”), 7.95 (2H, d, J= 20.4 Hz, H-3’, 5’), 7.58-7.51 (3H, m, H-6, 3”, 5”). 13C-NMR (DMSO, 100 MHz) δ: 165.0 (C, CO), 145.0 (C, C-3a, C-7a), 138.9 (C, C-1”), 137.7 (C, C-4’), 134.9 (C, C-1’), 132.2 (CH, C-3”, C-5”), 128.7 (CH, C-2”, C-6”), 126.9 (CH, C-5, C-6), 121.1 (CH, C-2’, C-6’), 120.9 (CH, C-3’, C-5’), 120.2 (CH, C-4, C-7), 118.4 (C, CN), 116.6 (C, C-4”). LC/MS: m/z: 340 (M+H). Anal. Calcd (%) C20H13N5O: C, 70.79; H, 3.86; N, 20.64. Found C, 70.49; H, 3.52; N, 20.74.
2.7.4.8. N-(4-(2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-nitrobenzamide (15a)
Compound (15a) was obtained as described in the general procedure starting from 4a and 4-nitrobenzoyl chloride for 2h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: > 300°C (from EtOH). Yield, 15%. TLC (petroleum ether/ethyl acetate 1:1): Rf 0.91. 1H-NMR (DMSO, 400 MHz) δ: 10.90 (1H, s, NHCO), 8.41 (2H, d, J= 8.0 Hz, H-2’, 6’), 8.35 (2H, d, J= 8.4 Hz, H-2”, 6”), 8.24 (2H, d, J= 8.4 Hz, H-3’, 5’), 8.09 (2H, d, J= 8.8 Hz, H-3”, 5”), 8.08-8.00 (2H, m, H-4, 7), 7.58-7.50 (2H, m, H-5, 6). 13C-NMR (DMSO, 100 MHz) δ: 164.2 (C, CO), 149.3 (C, C-4”), 144.4 (C, C-3a, C-7a), 140.3 (C, C-4’), 139.8 (C, C-1”), 135.4 (C, C-1’), 129.3 (CH, C-2”, C-6”), 127.5 (CH, C-5, C-6), 123.6 (CH, C-3”, C-5”), 121.2 (CH, C-2’, C-6’), 120.8 (CH, C-3’, C-5’), 118.1 (CH, C-4, C-7). LC/MS: m/z: 360 (M+H). Anal. Calcd (%) C19H13N5O3: C, 63.51; H, 3.65; N, 19.49. Found: C, 63.40; H, 3.35; N, 19.60.
2.7.4.9. N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-methylbenzamide (8b)
Compound (8b) was obtained as described in the general procedure starting from 4b and 4-methylbenzoyl chloride for 24 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. white solid, m.p.: 295-296°C (from EtOH). Yield, 46%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.53. 1H-NMR (DMSO, 400 MHz) δ: 10.46 (1H, s, NH), 8.25 (2H, d, J= 9.2 Hz, H-2’, 6’), 8.05 (2H, d, J= 9.2 Hz, H-3’, 5’), 7.91 (2H, d, J= 8.4 Hz, H-2”, 6”), 7.76 (2H, s, H-4, 7), 7.37 (2H, d, J= 8.4 Hz, H-3”, 5”), 2.51 (3H, s, CH3), 2.40 (6H, s, 2CH3). 13C-NMR (DMSO, 100 MHz) δ: 165.6 (C, CO), 143.8 (C, C-3a, C-7a), 141.9 (C, C-4”), 139.9 (C, C-4’), 137.9 (C, C-5, C-6), 135.1 (C, C-1’), 131.7 (C, C-1”), 128.9 (CH, C-3”, C-5”), 127.7 (CH, C-2”, C-6”), 121.4 (CH, C-4, C-7), 120.9 (CH, C-2’, C-6’), 116.2 (CH, C-3’, C-5’), 29.9 (CH3, CH3-C4”), 21.0 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 357 (M+H) Anal. Calcd (%) C22H20N4O: C, 74.14; H, 5.66; N, 15.72. Found: C, 74.24; H, 5.80; N, 16.02.
2.7.4.10. N-(4-(5,6-dimethyl-2H-benzo[d] [1-3]triazol-2-yl)phenyl)-4-methoxybenzamide (9b)
Compound (9b) was obtained as described in the general procedure starting from 4b and 4-methoxybenzoyl chloride for 24 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Cream solid, m.p.: 285-287°C (from EtOH). Yield, 32%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.59. 1H-NMR (DMSO, 400 MHz) δ: 10.38 (1H, s, NH), 8.25 (2H, d, J= 8.0 Hz, H-2’, 6’), 8.05 (2H, d, J= 8.4 Hz, H-3’, 5’), 8.01 (2H, d, J= 7.6 Hz, H-2”,6”), 7.76 (2H, s, H-4, 7), 7,09 (2H, d, J= 8.0 Hz, H-3”, 5”), 3.86 (3H, s, OCH3), 2.40 (6H, s, 2CH3). 13C-NMR (DMSO, 100 MHz) δ: 165.1 (C, CO), 162.1 (C, C-4”), 143.8 (C, C-3a, C-7a), 140.0 (C, C-4’), 137.8 (C, C-5, C-6), 134.9 (C, C-1’), 129.7 (CH, C-2”, C-6”), 126.6 (C, C-1”), 120.8 (CH, C-4, C-7), 120.3 (CH, C-2’, C-6’), 116.2 (CH, C-3’, C-5’), 113.6 (CH, C-3”, C-5”), 55.4 (OCH3), 20.4 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 373 (M+H). Anal. Calcd (%) C22H20N4O2: C, 70.95; H, 5.41; N, 15.04. Found: C, 70.85; H, 5.40; N, 15.44.
2.7.4.11. N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3,4,5-trimethoxybenzamide (10b)
Compound (10b) was obtained as described in the general procedure starting from 4b and 3,4,5-trimethoxybenzoyl chloride for 24 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. beige solid, m.p.: 272-273°C (from EtOH).Yield, 13%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.34. 1H-NMR (DMSO, 400 MHz) δ: 10.40 (1H, s, NH), 8.28 (2H, d, J= 8.8 Hz, H-2’,6’), 8.02 (2H, d, J= 9.2 Hz, H-3’, 5’), 7.76 (2H, s, H-2”, 6”), 7.32 (2H, s, H-4, 7), 3.90 (6H, s, 2OCH3), 3.75 (3H, s, OCH3), 2.41 (6H, s, 2CH3). 13C-NMR (DMSO, 100 MHz) δ: 165.1 (C, CO), 152.6 (C, C-3”, C-5”), 143.8 (C, C-3a, C-7a), 140.5 (C, C-4”), 139.7 (C, C-4’), 137.9 (C, C-5, C-6), 135.2 (C, C-1’), 129.7 (C, C-1”), 121.1 (CH, C-4, C-7), 120.3 (CH, C-2’, C-6’), 116.3 (CH, C-3’, C-5’), 105.4 (CH, C-2”, C-6”), 60.1 (OCH3-C4”), 56.1 (OCH3-C3”, OCH3-C5”,), 20.4 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 433 (M+H). Anal. Calcd (%) C24H24N4O4: C, 66.65; H, 5.59; N, 12.96. Found: C, 66.35; H, 5.42; N, 13.06.
2.7.4.12. 4-chloro-N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)benzamide (11b)
Compound (11b) was obtained as described in the general procedure starting from 4b and 4-chlorobenzoyl chloride for 2 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Whight solid, m.p.: >300°C (from EtOH). Yield, 37%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.25. 1H-NMR (DMSO, 400 MHz) δ: 10.61 (1H, s, NH), 8.27 (2H, d, J= 8.4 Hz, H-2’, 6’), 8.06-8.02 (4H, m, H-2”, 3”, 5”, 6”), 7.77 (2H, s, H-4, 7), 7.64 (2H, d, J= 8.4 Hz, H-3’, 5’), 2.41 (6H, s, 2CH3). 13C-NMR (DMSO, 100 MHz) δ: 153.6 (C, CO), 143.9 (C, C-3a, C-7a), 143.8 (C, C-4’), 138.8 (C, C-4”), 138.3 (C, C-5, C-6), 138.0 (C, C-1’), 137.9 (C, C-1”), 121.1 (CH, C-2”, C-6”), 120.1 (CH, C-3”, C-5”), 119.2 (CH, C-4, C-7), 116.3 (CH, C-2’, C-3’, C-5’, C-6’), 20.42 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 377 (M+H). Anal. Calcd (%) C21H17ClN4O: C, 66.93; H, 4.55; N, 14.87. Found: C, 66.83; H, 4.55; N, 15.00.
2.7.4.13. 4-bromo-N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)benzamide (12b)
Compound (12b) was obtained as described in the general procedure starting from 4b and 4-bromobenzoyl chloride for 24 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Whight solid, m.p.: >300°C (from EtOH). Yield, 28%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.58. 1H-NMR (DMSO, 400 MHz) δ: 10.43 (1H, s, NH), 8.36 (2H, s, H-4, 7), 8.23 (2H, d, J= 8.4 Hz, H-2’, 6’), 7.82 (2H, d, J= 8.0 Hz, H-3’, 5’), 7.76 (4H, s, H-2”, 3”, 5”, 6”), 2,40 (6H, s, 2-CH3). 13C-NMR (DMSO, 100 MHz) δ: 164.8 (C, CO), 143.8 (C, C-3a, C-7a), 139.6 (C, C-4’), 137.9 (C, C-5, C-6), 133.7 (C, C-4”), 131.5 (CH, C-3”, C-5”), 129.9 (CH, C-2”, C-6”), 125.6 (C, C-1’, C-1”), 121.0 (CH, C-4, C-7), 120.4 (CH, C-2’, C-6’), 116.3 (CH, C-3’, C-5’), 20.4 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 422 (M+H). Anal. Calcd (%) C21H17BrN4O: C, 59.87; H, 4.07; N, 13.30. Found: C, 60.87; H, 4.37; N, 13.55.
2.7.4.14. N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-(trifluoromethyl)benzamide (13b)
Compound (13b) was obtained as described in the general procedure starting from 4b and 4-(trifluoromethyl)benzoyl chloride for 24 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. white solid, m.p.: 295-297°C (from EtOH). Yield, 20%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.66. 1H-NMR (DMSO, 400 MHz) δ: 10.76 (1H, s, NH), 8.27 (2H, s, H-4, 7), 8.19 (2H, d, J= 8.0 Hz, H-2’, 6’), 8.06 (2H, t, H-3”, 5”), 7.94 (2H, d, J= 8.0, H-3’, 5’), 7.76 (2H, d, J= 10.0 Hz, H-2”, 6”), 2.40 (6H, s, 2CH3). 13C-NMR (DMSO, 100 MHz) δ: 164.6 (C, CO), 143.8 (C, C-3a, C-7a), 139.9 (C, C-4’), 139.4 (C, C-1”), 138.9 (C, C-1’), 137.9 (C, C-5, C-6), 135.0 (C, C-4”), 131.5 (C, CF3), 128.7 (CH, C-2”, C-6”), 125.4 (CH, C-3”, C-5”), 121.0 (CH, C-4, C-7), 120.4 (CH, C-2’, C-6’), 116.3 (CH, C-3’, C-5’), 20.4 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 411 (M+H). Anal. Calcd (%) C22H17F3N4O: C, 64.39; H, 4.18; N, 13.65. Found: C, 64.19; H, 4.20; N, 13.84.
2.7.4.15. 4-cyano-N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)benzamide (14b)
Compound (14b) was obtained as described in the general procedure starting from 4b and 4-cyanobenzoyl chloride for 24 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 294-297°C (from EtOH). Yield, 44%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.40. 1H-NMR (DMSO, 400 MHz) δ: 10.76 (1H, s, NH), 8.28 (2H, d, J= 9.2 Hz, H-2’, 6’), 8.14 (2H, d, J= 8.4 Hz, H-2”, 6”), 8.06-8.04 (4H, m, H-3’, 5’, 3”, 5”), 7.76 (2H, s, H-4, 7), 2.40 (6H, s, 2CH3). 13C-NMR (DMSO, 100 MHz) δ: 164.3 (C, CO), 143.8 (C, C-3a, C-7a), 139.3 (C, C-1”), 138.6 (C, C-4’), 137.9 (C, C-5, C-6), 135.5 (C, C-1’), 132.5 (CH, C-3”, C-5”) 128.6 (CH, C-2”, C-6”), 121.1 (CH, C-4, C-7), 120.4 (CH, C-2’, C-6’), 118.2 (C, CN), 116.3 (CH, C-3’, C-5’), 114.0 (C, C-4”), 20.41 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 368 (M+H). Anal. Calcd (%) C22H17N5O: C, 71.92; H, 4.66; N, 19.06. Found: C, 72.00; H, 4.76; N, 19.36.
2.7.4.16. N-(4-(5,6-dimethyl-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-nitrobenzamide (15b)
Compound (15b) was obtained as described in the general procedure starting from 4b and 4-nitrobenzoyl chloride for 2 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 297-298°C (from EtOH). Yield, 49%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.75. 1H-NMR (DMSO, 400 MHz) δ: 10.86 (1H, s, NH), 8.40 (2H, d, J= 9.2 Hz, H-3”, 5”), 8.30 (2H, d, J= 7.6 Hz, H-2’, 6’), 8.23 (2H, d, J= 7.6 Hz, H-3’, 5’), 8.06 (2H, d, J= 9.2 Hz, H-2”, 6”), 7.77 (2H, s, H-4, 7), 2.41 (6H, s, 2-CH3). 13C-NMR (DMSO, 100 MHz) δ: 165.8 (C, CO), 150.0 (C, C4”), 143.9 (C, C-3a, C-7a), 143.8 (C, C-4’), 138.2 (C, C-5, C-6), 138.0 (C, C-1”), 136.4 (C, C-1’), 130.7 (CH, C-2”, C-6”), 129.4 (CH, C-4, C-7), 123.7 (CH, C-3”, C-5”), 121.0 (CH, C-2’, C-6’), 116.3 (CH, C-3’, C-5’), 20.4 (CH3, CH3-C5, CH3-C6). LC/MS: m/z: 388 (M+H). Anal. Calcd (%) C21H17N5O3: C, 65.11; H, 4.42; N, 18.08. Found: C, 65.00; H, 4.12; N, 18.28.
2.7.4.17. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-methylbenzamide (8c)
Compound (8c) was obtained as described in the general procedure starting from 4c and 4-methylbenzoyl chloride for 72 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 249-250°C (from EtOH). Yield, 17%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.59. 1H-NMR (DMSO, 400 MHz) δ: 10.50 (1H, br s, NH), 8.32 (2H, d, J = 8 Hz, H-2”,6”), 8.10 (2H, d, J = 8.8 Hz, H-2'-6'), 7.93-7.83 (3H, m, H-3'-5' e H-7), 7.53-7.48 (1H, m, H-6), 7.48-.7.29 (3H, m, H-3”,5” and H-5), 2.40 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 165.8 (C, CO), 151.3 (C, C-4), 146.9 (C, C-7a), 146.3 (C, C-4”), 142.1 (C, C-4’), 140.5 (C, C-1’), 134.6 (C, C-3a), 131.4 (C, C-1”), 129.0 (CH, C-3”, C-5”), 127.7 (CH, C-2”, C-6”), 121.3 (CH, C-6), 121.0 (CH, C-2’, C-3’, C-5’, C-6’), 120.7 (CH, C-7), 115.2 (CH, C-5), 21.0 (CH3). LC/MS: m/z 347 (M+H). Anal. Calcd. (%) C20H15FN4O: C, 69.35; H, 4.37; N, 16.18. Found: C, 69.41; H, 4.43; N, 16.21.
2.7.4.18. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-methoxybenzamide (9c)
Compound (9c) was obtained as described in the general procedure starting from 4c and 4-methoxybenzoyl chloride for 168 h. The crude product was purified by silica gel column chromatography eluting with a 8:2 mixture of petroleum ether/ethyl acetate. Cream solid, m.p.: 223-225°C (from EtOH). Yield, 15%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.42. 1H-NMR (DMSO, 400 MHz): 10.42 (1H, br s, NH), 8.32 (2H, d, J = 8.4 Hz, H-2”, 6”), 8.06 (2H, d, J = 8.4 Hz, H-2', 6'), 8.01 (2H, d, J = 8 Hz, H-3'-5'), 7.88 (1H, d, J = 8.4 Hz, H-7), 7.53-7.48 (1H, m, H-6), 7.34 (1H, t, H-5), 7.10 (2H, d, J = 8 Hz, H-3”,5”), 2.56 (3H, s, OCH3). 13C-NMR (DMSO, 100 MHz) δ: 165.1 (C, CO). 162.1 (C, C-4), 151.5 (C, C-4“), 146.9 (C, C-7a), 144.3 (C, C-1‘), 140.9 (C, C-1“), 134.4 (C, C-3a), 129.7 (CH, C-2“, C-6“), 127.7 (CH, C-6), 126.5 (C, C-4‘), 121.0 (CH, C-2‘, C-6‘), 120.8 (CH, C-3‘, C-5‘), 118.0 (CH, C-7), 114.6 (CH, C-3“, C-5“), 112.6 (CH, C-5), 55.4 (OCH3). LC/MS: m/z 363 (M+H). Anal. Calcd. (%) C20H15FN4O2: C, 66.29; H, 4.17; N, 15.46. Found: C, 66.38; H, 4.25; N, 15.54.
2.7.4.19. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3,4,5-trimethoxybenzamide (10c)
Compound (10c) was obtained as described in the general procedure starting from 4c and 3,4,5-trimethoxybenzoyl chloride for 72 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Orange solid, m.p.: 216-219°C (from EtOH). Yield, 26%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.46. 1H-NMR (DMSO, 400 MHz) δ: 10.45 (1H, br s, NH), 8.35 (2H, d, J = 8.4 Hz, H-3',5'), 8.07 (2H, d, J = 8.4 Hz, H-2', 6'), 7.89 (1H, d, J = 8.8 Hz, H-7), 7.54-7.48 (1H, m, H-6), 7.37-7.34 (1H, m, H-5), 7.32 (2H, s, H-2”, 6”), 3.92 (6H, s, CH3), 3.755 (3H, s, CH3).13C-NMR (DMSO, 100 MHz) δ: 165.2 (C, CO), 152.6 (C, C-4), 151.5 (C, C-3”, C-5”), 146.9 (C, C-7a), 140.5 (C, C-4”), 135.0 (C, C-3a), 134.7 (C, C-1’), 129.6 (C, C-4’), 127.7 (CH, C-6), 121.1 (CH, C-2’, C-3’, C-5’, C-6’), 114.6 (CH, C-7), 110.8 (CH, C-5), 105.4 (CH, C-2”, C-6”), 60.1 (CH3, OCH3-C4”), 56.1 (CH3, OCH3-C3”, OCH3-C5”). LC/MS: m/z 423 (M+H). Anal. Calcd. (%) C22H19FN4O4: C, 62.55; H, 4.53; N, 13.26. Found: C, 62.61; H, 4.60; N, 13.30.
2.7.4.20. 4-chloro-N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)benzamide (11c)
Compound (11c) was obtained as described in the general procedure starting from 4c and 4-chlorobenzoyl chloride for 192 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Wight solid, m.p.: 281-283°C (from EtOH). Yield, 69%. TLC (petroleum ether/ethyl acetate 8:2): Rf 0.43. 1H-NMR (DMSO, 400 MHz) δ: 10.63 (1H, br s, NH), 8.33 (2H, d, J = 8.8 Hz, H-2”,6”), 8.08(2H, d, J = 8.8 Hz, H-3”, 5”), 8.03 (2H, d, J = 8 Hz, H-3', 5'), 7.92 (1H, s, H-7), 7.63 (2H, d, J = 8 Hz, H-2', 6'), 7.54-7.51 (2H, m, H-5,6).13C-NMR (DMSO, 100 MHz) δ: 164.7 (C,CO), 145.2 (C, C-4), 144.4 (C, C-7a), 140.0 (C, C-1’), 137.6 (C, C-4”), 136.6 (C, C-1”), 135.1 (C, C-3a), 133.3 (C, C-4’), 129.7 (CH, C-3”, C-5”), 128.5 (CH, C-2”, C-6”), 127.5 (CH, C-6), 121.1 (CH, C-7), 121.0 (CH, C-2’, C-6’), 120.7 (CH, C-3’, C-5’), 118.1 (CH, C-5). LC/MS: m/z 369 (M+H). Anal. Calcd. (%) C19H12ClFN4O: C, 62.22; H, 3.30; N, 15.28. Found: C, 62.30; H, 3.38; N, 15.34.
2.7.4.21. 4-bromo-N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)benzamide (12c)
Compound (12c) was obtained as described in the general procedure starting from 4c and 4-bromobenzoyl chloride for 168 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Wight solid, m.p.: 285-286°C (from EtOH). Yield, 9%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.60. 1H-NMR (DMSO, 400 MHz) δ: 10.64 (1H, br s, NH), 8.32 (2H, d, J = 7.6 Hz, H-2”,6”), 8.07 (2H, d, J = 7.6 Hz, H-3”,5”), 8.03 (1H, d, J = 7.4 Hz, H-7), 7.96 (2H, d, J = 7.6 Hz, H-3',5'), 7.78 (2H, d, J = 7.6 Hz, H-2'-6'), 7.53-7.51 (1H, m, H-6), 7.34 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 164.9 (C, CO). 151.5 (C, C-4), 144.3 (C, C-7a), 140.0 (C, C-1’), 139.8 (C, C-1”), 135.2 (C, C-3a), 133.5 (C, C-4’), 131.5 (CH, C-3”, C-5”), 129.8 (CH, C-2”. C-6”), 127.6 (CH, C-6), 125.6 (C, C-4”), 121.1 (CH, C-7), 121.0 (CH, C-2’, C-6’), 120.8 (CH, C-3’, 5’), 118.0 (CH, C-5). LC/MS: m/z 412 (M+H). Anal. Calcd. (%) C19H12BrFN4O: C, 55.49; H, 2.94; N, 13.62. Found: C, 55.53; H, 2.98; N, 13.68.
2.7.4.22. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-(trifluoromethyl)benzamide (13c)
Compound (13c) was obtained as described in the general procedure starting from 4c and 4-(trifluoromethyl)benzoyl chloride for 72 h. The crude product was purified by silica gel column chromatography eluting with a 8:2 mixture of petroleum ether/ethyl acetate. Beige solid, m.p.: 218-220°C (from EtOH). Yield, 22%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.15. 1H-NMR (DMSO, 400 MHz) δ: 10.79 (1H, 1, br s, NH), 8.32 (2H, d, J = 8.8 Hz, H- 2”, 6”), 8.15 (2H, d, J = 7.6 Hz, H-3'-5'), 8.07-7.10 (3H, m, H-3”,5”, H-7), 7.92 (2H, d, J = 7.6 Hz, H-2',6'), 7.52-7.47 (1H, m, H-6), 7.32 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 164.8 (C, CO). 151.4 (C, C-4), 144.3 (C, C-7a), 139.8 (C, C-1‘), 138.3 (C, C-1“), 135.1 (C, C-3a), 131.6 (C, C-4“), 128.6 (CH, C-2“, C-6“), 127.6 (CH, C-6), 125.4 (CH, C-7), 125.2 (C, C-1“), 122.5 (C, CF3), 121.1 (CH, C-3“, C-5“), 120.8 (CH, C-2‘, C-6‘), 118.0 (CH, C-3‘, C-5‘), 112.6 (CH, C-5). LC/MS: m/z 401 (M+H). Anal. Calcd. (%) C20H12F4N4O: C, 60.00; H, 3.02; N, 14.00. Found: C, 60.09; H, 3.08; N, 14.10.
2.7.4.23. 4-cyano-N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)benzamide (14c)
Compound (14c) was obtained as described in the general procedure starting from 4c and 4-cyanobenzoyl chloride for 144 h. The crude product was purified by silica gel column chromatography eluting with a 6:4 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 234-235°C (from EtOH). Yield, 21%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.20. 1H-NMR (DMSO, 400 MHz) δ: 10.57 (1H, br s, NH), 8.34 (1H, s, H-7), 8.26 (2H, d, J= 8.8 Hz, H-3”,5”), 7.98 (2H, d, J = 6.8 Hz, H-2', 6'),7.84 (2H, d, J = 8.8 Hz, H-2”-6”), 7.50 (2H, d, J = 6.8 Hz, H-3'-5'), 7.32-7.28 (1H, m, H-6), 7.30 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 151.5 (C, CO), 146.9 (C, C-4), 144.3 (C, C-7a), 139.6 (C, C-1’), 139.5 (C, C-1”), 139.2 (C, C-4’), 135.2 (C, C-3a), 135.0 (C, CN), 134.6 (C, C-4”), 127.5 (CH, C-3”, C-5”), 121.5 (CH, C-6), 121.3 (CH, C-7), 121.0 (CH, C-2”, C-6”), 119.9 (CH, C-2’, C-6’), 118.0 (CH, C-3’, C-5’), 118.0 (CH, C-5). LC/MS: m/z 358 (M+H). Anal. Calcd. (%) C20H12FN5O: C, 67.22; H, 3.38; N, 19.60. Found: C, 67.31; H, 3.43; N, 19.70.
2.7.4.24. N-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-4-nitrobenzamide (15c)
Compound (15c) was obtained as described in the general procedure starting from 4c and 4-nitrobenzoyl chloride for 144 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Light yellow solid, m.p.: 315-317°C (from EtOH). Yield, 79%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.63. 1H-NMR (DMSO, 400 MHz) δ: 10.88 (1H, br s, NH), 8.39 (2H, d, J = 8.4 Hz, H-2”,6”), 8.36-8.33 (2H, m, H-2',6'), 8.22 (2H, d, J = 8.4 Hz, H-3”, 5”),8.11-8.01 (2H, m, H-3',5'), 7.88 (1H, d, J = 8.4 Hz, H-7), 7.53-7.48 (1H, m, H-6),7.34 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 164.2 (C, CO), 151.5 (C, C-4), 146.9 (C, C-7a), 144.4 (C, C-NO2), 140.2 (C, C-4’), 139.8 (C, C-1”), 135.3 (C, C-3a), 135.0 (C, C-1’), 129.0 (CH, C-2”, C-6”), 127.7 (CH, C-6), 123.6 (CH, C-3”, C-5”), 121.1 (CH, C-2’, C-6’), 121.1 (CH, C-7), 120.8 (CH, C-3’, C-5’), 110.8 (CH, C-5). LC/MS: m/z 378 (M+H). Anal. Calcd. (%) C19H12FN5O3: C, 60.48; H, 3.21; N, 18.56. Found: C, 60.52; H, 3.28; N, 18.62.
2.7.5. General procedure for the preparation of 3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)ureaderivatives (16c-26c).
To a solution of 4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)aniline (4c) (200 mg, 0.87 mmol) in N,N-dimethylacetamide (DMA, 7 ml) or toluene (7 ml) an excess (2.61 mmol) of the required isocyanate derivatives was added. The resulting solution was stirred at 110°C for different times as reported below, then it was cooled to room temperature and crushed ice was added. The solid obtained in each reaction was filtered under vacuum. Purification by flash chromatography using as eluting system, a mixture of petroleum ether/ethyl acetate or dichloromethane/ethyl acetate in different ratios as reported below, furnished the urea derivatives 16c-26c.
2.7.5.1. 1-ethyl-3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)urea (16c)
Compound (16c) was obtained as described in the general procedure starting from 4c and ethyl isocyanate in DMA for 120 h. The crude product was purified by silica gel column chromatography eluting with a 1:1 mixture of petroleum ether/ethyl acetate. White solid, m.p.: 345-348°C (from EtOH). Yield, 38%. TLC (petroleum ether/ethyl acetate 1:1): Rf 0.38. 1H-NMR (DMSO, 400 MHz) δ: 8.86 (1H, br s, NH), 8.19 (2H, d, J = 7.6 Hz, H-2',6'), 7.86 (1H, d, J = 7.4 Hz, H-7), 7.68 (2H, d, J = 8.8 Hz, H-3',5'), 7.47-7.51 (1H, m, H-6), 7.32 (1H, t, H-5), 6.27 (1H, br t, NH), 3.15 (2H, m, CH2), 1.08 (3H, t, CH3). 13C-NMR (DMSO, 100 MHz) δ: 154.8 (C, CO), 151.4 (C, C-4), 146.8 (C, C-7a), 142.1 (C, C-1‘), 135.2 (C, C-3a), 132.6 (C, C-4‘), 127.4 (CH, C-6), 121.0 (CH, C-3‘, C-5‘), 118.4 (CH, C-2‘, C-6‘), 114.4 (CH, C-7), 110.5 (CH, C-5), 34.0 (CH2, CH2CH3), 15.3 (CH3, CH2CH3). LC/MS: m/z 300 (M+H). Anal. Calcd. (%) C15H14FN5O: C, 60.19; H, 4.71; N, 23.40. Found: C, 60.32; H, 4.81; N, 23.51.
2.7.5.2. 1-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3-propylurea (17c)
Compound (17c) was obtained as described in the general procedure starting from 4c and 1-propyl isocyanate in DMA for 72 h. The crude product was purified by silica gel column chromatography eluting with a 6:4 mixture of petroleum ether/ethyl acetate. Cream solid, m.p.: 321-324°C (from EtOH). Yield, 46%. TLC (petroleum ether/ethyl acetate 6:4): Rf 0.32. 1H-NMR (DMSO, 400 MHz) δ: 8.84 (1H, br s, NH), 8.19 (2H, d, J = 7.6 Hz, H-2',6'), 7.86 (1H, d, J = 7.4 Hz, H-7), 7.68 (2H, d, J = 8.8 Hz, H-3',5'), 7.50-7.45 (1H, m, H-6), 7.31 (1H, t, H-5),6.30 (1H, br t, NH), 3.08 (2H, q, CH2), 1.47 (2H, sext, CH2), 0.89 (3H, t, CH3). 13C-NMR (DMSO, 100 MHz) δ: 155.0 (C, CO), 151.4 (C, C-4), 146.8 (C, C-7a), 142.2 (C, C-1‘), 135.1 (C, C-3a), 133.5 (C, C-4‘), 127.5 (CH, C-6), 121.2 (CH, C-3‘, C-5‘), 118.2 (CH, C-2‘, C-6‘), 114.4 (CH, C-7), 110.5 (CH, C-5), 40.9 (CH2, COCH2), 22.8 (CH2, CH2CH3), 11.2 (CH3, CH2CH3). LC/MS: m/z 314 (M+H). Anal. Calcd. (%) C16H16FN5O: C, 61.33; H, 5.15; N, 22.35. Found: C, 61.42; H, 5.20; N, 22.42.
2.7.5.3. 1-butyl-3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)urea (18c)
Compound (18c) was obtained as described in the general procedure starting from 4c and 1-butyl isocyanate in DMA for 96 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Brown solid, m.p.: 243-245°C (from EtOH). Yield, 51%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.42. 1H-NMR (DMSO, 400 MHz) δ: 8.84 (1H, br s, NH), 8.18 (2H, d, J = 8.8 Hz, H-3̍, 5̍), 7.85 (1H, d, J= 8.4 Hz, H-7), 7.67 (2H, d, J = 8.8 Hz, H-2̍, 6̍), 7.50-7.45 (1H, m, H-6), 7.31 (1H, t, H-5), 6.28 (1H, br t, NH), 3.12 (2H, d, CH2), 1.44 (2H, q, CH2), 1.33 (2H, sex, CH2), 0.90 (3H, t, CH3).13C-NMR (DMSO, 100 MHz) δ: 154.9 (C, CO), 151.3 (C, C-4), 146.7 (C, C-7a), 142.0 (C, C-1‘), 135.1 (C, C-3a), 132.6 (C, C-4‘), 127.5 (CH, C-6), 121.1 (CH, C-3‘, 5‘), 118.0 (CH, C-2‘, C-6‘), 116.3 (CH, C-7), 112.4 (CH, C-5), 39.2 (CH2, COCH2), 31.6 (CH2, CH2CH2CH2), 19.4 (CH2, CH2CH3), 13.6 (CH3, CH2CH3). LC/MS: m/z 328 (M+H). Anal. Calcd. (%) C17H18FN5O: C, 62.37; H, 5.54; N, 21.39. Found: C, 62.41; H, 5.67; N, 21.43.
2.7.5.4. 1-(sec-butyl)-3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)urea (19c)
Compound (19c) was obtained as described in the general procedure starting from 4c and sec-butyl isocyanate in DMA for 144 h. The crude product was purified by silica gel column chromatography eluting with a 75:25 mixture of petroleum ether/ethyl acetate. White solid, m.p.: 252-255°C (from EtOH). Yield, 42%. TLC (petroleum ether/ethyl acetate 75:25): Rf 0.32. 1H-NMR (DMSO, 400 MHz) δ: 8.71 (1H, br s, NH), 8.17 (2H, d, J = 8.8 Hz, H-3̍, 5̍), 7.86 (1H, d, J= 8.4 Hz, H-7), 7.42 (2H, d, J = 8.8 Hz, H-2̍, 6̍), 7.34-7.30 (1H, m, H-6), 7.31 (1H, t, H-5), 6.128 (1H, br t, NH), 3.63 (1H, sept, CH), 1.45 (2H, quin, CH2), 1.10 (3H, d, CH3), 0.89(3H, t, CH3). 13C-NMR (DMSO, 100 MHz) δ: 152.7 (C, CO), 148.5 (C, C-4), 146.8 (C, C-7a), 144.2 (C, C-1‘), 142.0 (C, C-3a), 132.7 (C, C-4‘), 127.3 (CH, C-6), 121.2 (CH, C-3‘, 5‘), 120.9 (CH, C-7), 117.9 (CH, C-2‘, C-6‘), 114.4 (CH, C-5), 46.2 (CH, CH-CH3), 29.2 (CH2, CH2-CH3), 20.6 (CH3, CH-CH3), 10.3 (CH3, CH2-CH3). LC/MS: m/z 328 (M+H). Anal. Calcd. (%) C17H18FN5O: C, 62.37; H, 5.54; N, 21.39. Found: C, 62.42; H, 5.60; N, 21.44.
2.7.5.5. 1-cyclopentyl-3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)urea (20c)
Compound (20c) was obtained as described in the general procedure starting from 4c and cyclopentyl isocyanate in DMA for 168 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Cream solid, m.p.: 339-341°C (from EtOH). Yield, 23%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.27. 1H-NMR (DMSO, 400 MHz) δ: 8.71 (1H, br s, NH), 8.17 (2H, d, J = 8.8 Hz, H-3̍, 5̍), 8.01 (1H, s, H-7), 7.65 (2H, d, J = 8.8 Hz, 2̍, 6̍), 7.50-7.49 (2H, m, H-5), 6.32 (1H, br d, NH), 3.00-3.97 (1H, m, CH cyclopentyl), 1.87-1.82 (2H, m, CH2 cyclopentyl), 1.65-1.55 (4H, m, CH2, cyclopentyl), 1.40-1.39 (2H, m, CH2 cyclopentyl). 13C-NMR (DMSO, 100 MHz) δ: 154.6 (C, CO). 144.7 (C, C-4), 144.2 (C, C-7a), 142.0 (C, C-1‘), 139.4 (C, C-4‘), 133.0 (C, C-3a), 127.2 (CH, C-6), 121.0 (CH, C-3‘, C-5‘), 117.9 (CH, C-2‘, C-6‘), 114.4 (CH, C-7), 110.6 (CH, C-5), 50.9 (CH, CH-NH), 32.7 (CH2,CH2-CH), 23.1 (CH2, CH2-CH), 22.5 (CH2, CH2-CH2), 21.2 (CH2, CH2-CH2). LC/MS: m/z 340 (M+H). Anal. Calcd. (%) C18H18FN5O: C, 63.70; H, 5.35; N, 20.64. Found: C, 63.81; H, 5.43; N, 20.70.
2.7.5.6. 1-cyclohexyl-3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)urea (21c)
Compound (21c) was obtained as described in the general procedure starting from 4c and cyclohexyl isocyanate in DMA for 72 h. The crude product was purified by silica gel column chromatography eluting with a 7:3 mixture of petroleum ether/ethyl acetate. Cream solid, m.p.: 212-214°C (from EtOH). Yield, 86%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.46. 1H-NMR (DMSO, 400 MHz) δ: 8.73 (1H, br s, NH), 8.28 (2H, d, J = 8.4 Hz, H-3̍, 5̍), 7.86 (1H, d, J= 8.8 H-7), 7.66 (2H, d, J = 8.4, H-2̍, 6̍), 7.51-7.46 (1H, m, H-6), 7.36- 7.29 (1H, m, H-5), 6.23 (1H, br d, NH), 1.84-1.49 (6H, m, H-cyclohexyl), 1.37-1.11 (5H, m, H- cyclohexyl). 13C-NMR (DMSO, 100 MHz) δ: 156.6 (C, CO). 154.1 (C, C-4), 151.4 (C, C-1‘), 146.8 (C, C-7a), 142.2 (C, C-4‘), 135.2 (C, C-3a), 127.4 (CH, C-6), 121.2 (CH, C-3‘, C-5‘), 117.8 (CH, C-2‘, C-6‘), 114.4 (CH, C-7), 110.5 (CH, C-5), 47.6 (CH, CH-NH), 33.3 (CH2, CH2-CH), 32.8 (CH2, CH2-CH), 25.2 (CH2), 24.3 (CH2), 22.4 (CH2). LC/MS: m/z 354 (M+H). Anal. Calcd. (%) C19H20FN5O: C, 64.57; H, 5.70; N, 19.82. Found: C, 64.61; H, 5.79; N, 19.88.
2.7.5.7. 1-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3-(naphthalen-1-yl)urea (22c)
Compound (22c) was obtained, pure, as described in the general procedure starting from 4c and 1-naphthylisocyanate in DMA for 24 h. White solid, m.p.: >350°C (from EtOH). Yield, 40%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.36. 1H-NMR (DMSO, 400 MHz) δ: 9.47 (1H, br s, NH), 8.90 (1H, br s, NH), 8.28 (2H, d, J = 8.8 Hz, H-2', 6'), 8.14 (1H, d, J = 8.4 Hz, H-naphthalene), 8.02-7.95 (3H, m, H-6, 2H-naphthalene), 7.88 (1H, d, J= 8.4 Hz, H-7), 7.80 (2H, d, J = 8.8 Hz, H-3', 5'), 7.70-7.48 (4H, m, H-naphthalene), 7.33 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 152.9 (C, CO). 152.7 (C, C-4), 150.1 (C, C-7a), 144.2 (C, C-1“), 141.2 (C, C-4‘), 135.2 (C, C-3a), 133.7 (C, C-4a“), 133.3 (C, C-1‘), 128.4 (CH, C-5“, 7“), 127.6 (CH, C-6), 126.3 (C, C-8a“), 126.0 (CH, C-3“, C-6“), 123.6 (CH, C-2‘, C-6‘), 123.6 (CH, C-4“), 118.7 (CH, C-3‘, C-5‘), 117.9 (CH, C-8“), 118.4 (CH, C-7), 114.4 (CH, C-5), 110.6 (CH, C-2“). LC/MS: m/z 398 (M+H). Anal. Calcd. (%) C23H16FN5O: C, 69.51; H, 4.06; N, 17.62. Found: C, 69.82; H, 4.25; N, 17.71.
2.7.5.8. 1-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3-(4-methoxyphenyl)urea (23c)
Compound (23c) was obtained as described in the general procedure starting from 4c and 4-methoxyphenyl isocyanate in toluene for 96 h. The crude product was purified by silica gel column chromatography eluting with a 9.5:0.5 mixture of dichloromethane/ethyl acetate. Light pink solid, m.p.: 274-276°C (from EtOH). Yield, 33%. TLC (dichloromethane/ethyl acetate 9.5:0.5): Rf 0.36. 1H-NMR (DMSO, 400 MHz) δ: 9.08 (1H, br s, NH), 8.67 (1H, br s, NH),8.25 (2H, d, J = 8.8 Hz, H-2', 6'), 7.88 (1H, d, J= 8.8 Hz H-7), 7.74 (2H, d, J = 9.2 Hz, H-2”, 6”),7.53-7.47 (1H, m, H-6), 7.40 (2H, d, J = 8.8 Hz, H-3', 5'), 7.36- 7.31 (1H, m, H-5), 6.90 (2H, d, J = 9.2 Hz, H-3”, 5”),3.73 (3H, s, OCH3). 13C-NMR (DMSO, 100 MHz) δ: 156.0 (C, CO), 154.8 (C, C-4), 152.6 (C, C-4“), 152.4 (C, C-7a), 141.3 (C, C-1‘), 136.0 (C, C-3a), 132.2 (C, C-1“), 132.0 (C, C-4‘), 127.7 (CH, C-6), 121.3 (CH, C-2“, C-6“), 120.4 (CH, C-3‘, C-5‘), 118.6 (CH, C-2‘, C-6‘), 114.5 (CH, C-3“, C-5“), 114.0 (CH, C-7), 110.6 (CH, C-5), 55.2 (CH3, OCH3). LC/MS: m/z 378 (M+H). Anal. Calcd. (%) C20H16FN5O2: C, 63.65; H, 4.27; N, 18.56. Found: C, 63.71; H, 4.34; N, 18.65.
2.7.5.9. 1-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3-(3,4,5-trimethoxyphenyl)urea (24c)
Compound (24c) was obtained, pure, as described in the general procedure starting from 4c and 3, 4, 5-trimethoxyphenyl isocyanate in toluene for 20 h. White solid, m.p.: 272-274°C (from EtOH). Yield, 73%. TLC (petroleum ether/ethyl acetate 1:1): Rf 0.32. 1H-NMR (DMSO, 400 MHz) δ: 9.06 (1H, br s, NH), 8.77 (1H, br s, NH), 8.20 (2H, d, J = 8.8 Hz, H-2', 6'),7.83 (1H, d, J= 8.4 Hz H-7), 7.70 (2H, d, J = 8.8 Hz, H-3', 5'), 7.51- 7.46 (1H, m, H-6), 7.29 (1H, m, H-5), 6.80 (2H, s, H-2”,6”), 3.77 (6H, s, OCH3), 3.60 (3H, s, OCH3). 13C-NMR (DMSO, 100 MHz) δ: 152.8 (C, CO), 152.6 (C, C-4), 150.1 (C, C-3“, C-5“), 146.8 (C, C-7a), 140.9 (C, C-1“), 140.5 (C, C-1‘), 140.2 (C, C-4“), 135.1 (C, C-3a), 133.4 (C, C-4‘), 121.3 (CH, C-6), 119.0 (CH, C-3‘, C-5‘), 117.8 (CH, C-7), 114.4 (CH, C-2‘, C-6‘), 110.7 (CH, C-2“, C-6“), 96.3 (CH, C-5), 60.2 (CH3, OCH3-C4”), 55.7 (CH3, OCH3-C3”, OCH3-C5”). LC/MS: m/z 438 (M+H). Anal. Calcd. (%) C22H20FN5O4: C, 60.41; H, 4.61; N, 16.01. Found: C, 60.51; H, 4.67; N, 16.10.
2.7.5.10. 1-(4-chlorophenyl)-3-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)urea (25c)
Compound (25c) was obtained, pure, as described in the general procedure starting from 4c and 4-chlorophenyl isocyanate in toluene for 3.5 h. White solid, m.p.: 241-244°C (from EtOH). Yield, 63%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.20. 1HNMR (DMSO, 400 MHz) δ: 9.06 (1H, br s, NH), 8.91 (1H, br s, NH), 8.23 (2H, d, J = 8.8 Hz, H-6”,5”), 8.09-8.06 (1H, m, H-6), 7.94 (1H, d, J= 8.4 Hz, H-7), 7.81 (2H, d, J = 8.8 Hz, H-2”, 6”), 7.58 (2H, d, J = 8.8 Hz, H-2', 6'), 7.41 (2H, d, J = 8.8 Hz, H-3', 5'), 7.29 (1H, t, H-5). 13C-NMR (DMSO, 100 MHz) δ: 153.1 (C, CO), 151.6 (C, C-4), 147.3 (C, C-7a), 144.7 (C, C-1‘), 141.4 (C, C-1“), 138.5 (C, C-3a), 133.9 (C, C-4“), 129.1 (CH, C-3“, C-5“), 126.31 (C, C-4‘), 121.8 (CH, C-6), 120.5 (CH, C-2“, C-6“), 119.3 (CH, C-3‘, C-5‘), 118.4 (CH, C-2‘, C-6‘), 114.9 (CH C-7), 111.2 (CH, C-5). LC/MS: m/z 384 (M+H). Anal. Calcd. (%) C19H13ClFN5O: C, 59.77; H, 3.43; N, 18.34. Found: C, 59.86; H, 3.62; N, 18.42.
2.7.5.11. 1-(4-(4-fluoro-2H-benzo[d] [1-3] triazol-2-yl)phenyl)-3-(4-fluorophenyl)urea (26c)
Compound (26c) was obtained, pure, as described in the general procedure starting from 4c and 4-fluorophenyl isocyanate in toluene for 48 h. White solid, m.p.: 346-347°C (from EtOH). Yield, 38%. TLC (petroleum ether/ethyl acetate 7:3): Rf 0.47. 1H-NMR (DMSO, 400 MHz) δ: 9.05 (1H, br s, NH), 8.80 (1H, br s, NH), 8.22 (2H, d, J = 8.0 Hz, H-2', 6'), 7.84 (1H, d, J= 8.8 Hz, H-7), 7.72 (2H, d, J = 8.0 Hz, H-3', 5'),7.49- 7.46 (3H, m, H-6, 2”, 6”), 7.29 (1H, t, H-5), 7.13 (2H, t, H-3”,5”). 13C-NMR (DMSO, 100 MHz) δ: 158.0 (C, CO), 152.9 (C, C-4), 151.9 (C, C-4“), 147.3 (C, C-7a), 144.7 (C, C-1‘), 144.8 (C, C-1“), 136.2 (C, C-3a), 133.8 (C, C-4‘), 121.7 (CH, C-2“, C-6“), 120.7 (CH, C-6), 119.2 (CH, C-3‘, C-5‘), 115.9 (CH, C-2‘, 6‘), 115.6 (CH, C-3“, C-5“), 115.0 (CH, C-7), 111.1 (CH, C-5). LC/MS: m/z 366 (M+H). Anal. Calcd. (%) C19H13F2N5O: C, 62.46; H, 3.59; N, 19.17. Found: C, 62.53; H, 3.65; N, 19.26.
3. RESULTS AND DISCUSSION
3.1. Chemistry
Amines 4a,b were prepared according to the procedures we have already reported [23] while N-(4-nitrophenyl) derivatives (1-3) and N-(4-aminophenyl) derivative (4c) were synthesized starting from 4-fluorobenzotriazole as previously described [46] and 1-chloro-4-nitrobenzene in dimethylacetamide (DMA) and Cs2CO3, to afford the desired 4-fluoro-2-(4-nitrophenyl)-2H-benzo[d] [1-3]triazole (2) in 37% yield and traces of 7-fluoro-1-(4-nitrophenyl)-1H-benzo[d] [1-3]triazole (1) and 4-fluoro-1-(4-nitrophenyl)-1H-benzo[d] [1-3]triazole (3) in 1-2% of yield. The reduction of derivative 2, dissolved in ethanol, with hydrazine hydrate in the presence of 10% Palladium on activated charcoal gave the desired 4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)aniline (4c) in over 50% of yield Scheme. (1).
As shown in Supplementary Material, the correct position of the side chain on the triazole moiety of intermediates 1-3, has been determined by analysis of the chemical shifts of C-3a, C-7a and C-1’, as previously reported [24], and verified with bidimensional techniques (NOESY and HMBC), see Figure SM3-SM11.
As reported in Scheme. (2), condensation of 4c with the appropriate anhydride (acetic, propionic or butyric anhydride), afforded the corresponding amides (5-7) in 26-31% of yield. While the condensation of 4c, in N,N-dimethylacetamide or toluene, with either required benzoyl chloride or isocyanate derivatives, afforded the N-(4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)phenyl)benzamide derivatives (8c-15c) in 9-79% of yield and 3-(4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)phenyl)urea derivatives (16c-26c) in 23-86% of yield, respectively. Condensation of compounds 4a,b with benzoyl chloride leads to the formation of derivatives 8a,b-15a,b.
3.2. Biology
Derivatives (5c-7c), (8a,b,c-15a,b,c) and (16c-26c), depicted in Scheme. (II), were tested against representative members of several RNA and DNA viruses families in cell-based assays (results reported in Supplementary Material, Table SM1). In detail, ssRNA- viruses: Vesicular Stomatitis Virus (VSV) (Rhabdoviridae) and human respiratory syncytial virus (hRSV) (Pneumoviridae); ssRNA+ viruses: BVDV and YFV (Flaviviridae) and two Picornaviridae: human enterovirus B (Coxsackievirus B5, CV-B5) human enterovirus C (poliovirus type-1, Sb-1); dsRNA viruses: reovirus type-1 (Reo-1) (Reoviridae); DNA virus: human herpesvirus 1 (herpes simplex type-1, HSV-1) (Herpesviridae) and vaccinia virus (VV) (Poxviridae). As reference drugs were used 2'-C-methylcytidine (NM 107), ribavirin, 6-azauridine, acycloguanosine (ACG), pleconaril and mycophenolic acid (MPA). Furthermore, the cytotoxicity of all compounds was also evaluated in parallel with the antiviral activity.
Results are presented and divided into three groups based on chemical similarities. As far as the antiviral activity is concerned, compounds belonging to N-(4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl)phenyl)amide derivatives (5c-7c) proved to be endowed with selective anti-enterovirus activity, as well as the 4-(4-fluoro-2H-benzo[d] [1-3]triazol-2-yl) aniline parent (4c), EC50 ranged between 3.8 and 50 µM, as shown in Table 1. These antiviral activity results turned out not far from the previously studied parental compounds either with two methyl groups in positions 5 and 6 (5b-7b) or without any substituent (5a-7a), on the benzotriazole moiety [15], which were reported in the same Table 1 to better compare the activities. Of the last compounds, we were able to show the compound/Polio helicase complexes, by in silico assays and we provided an insight into the interactions occurring in the active site [15]. We have also observed that the substitution of the methyl groups with chlorine atoms, in position 5 and 6 on the benzotriazole scaffold (5d-7d), causes a completely loss of activity, with both EC50 and CC50 values higher than 100 µM [16]. The newly synthesised derivatives with a fluorine atom in position 4 showed an increase in the antiviral activity; they have more widespread activities against CV-B5 and Sb-1 if compared to previously reported derivatives and turned out with lower EC50 values, combined with a low or no cytotoxicity.
| Compounds | Vero76 | CV-B5 | Sb-1 | Ref. |
|---|---|---|---|---|
| - | aCC50 | bEC50 | - | - |
| 4a | 60 | >60 | 42 | [15] |
| 4b | >100 | >100 | >100 | [15] |
| 4c | >100 | 42 | 19 | - |
| 4d | >100 | >100 | >100 | [16] |
| 5a | 71 | 28 | >71 | [15] |
| 5b | >100 | >100 | >100 | [15] |
| 5c | >100 | 41 | 50 | - |
| 5d | >100 | >100 | >100 | [16] |
| 6a | >100 | 30 | 47 | [15] |
| 6b | >100 | 10 | >100 | [15] |
| 6c | >100 | 11 | 37 | - |
| 6d | >100 | >100 | >100 | [16] |
| 7a | >100 | >100 | >100 | [15] |
| 7b | >100 | 30 | 53 | [15] |
| 7c | >100 | 10.2 | 3.8 | - |
| 7d | >100 | >100 | >100 | [16] |
| Ref. compounds | - | - | - | - |
| Pleconaril | 77±6.8 | 0.005±0.002 | 2±0.6 | - |
As shown in Table 1, the antiviral activity against the CV-B5 and Sb-1 for derivatives 5c-7c, increases with the size of the side chain, with compound 7c being the most active against both viruses.
Moreover, the substitution of the aliphatic side chain with an aromatic moiety on the amide nitrogen (compounds 8a,b,c-15a,b,c) dramatically cancels the antiviral activity (not listed in a table); on the contrary, the corresponding chlorine-derivatives have shown extensive antiviral activity against CV-B5, BVDV, hRSV [16] and orthohantavirus (HTNV) [25] with EC50 values ranged between 3 and 27 µM.
Emboldened by the high anti-hRSV activity of 5,6-dichloro-2-phenyl-benzotriazole urea derivatives [16], we have synthesised and tested a series of ureidic derivatives bearing the fluorine atom in position 4 (16c-26c). Surprisingly, none of the newly synthesised compounds 16c-26c showed antiviral activity against hRSV. On the other hand, they have once again proved to be active against CV-B5 and Sb-1, as shown in Table 2. Further, only derivatives bearing an aliphatic side chain were proved active against the virus replication. The potency increases as the chain length up to a maximum of 4 linear carbon atoms (compound 18c showed an EC50 value of 5.5 µM). Among urea derivatives already published for their antiviral activity against orthohantavirus (16d-18d, 20d-26d) [25], hRSV (16d-18d, 20d-26d) [16] and CV-B5 (16a,b-26a,b) [17], compounds 16d-18d, 20d-26d had turned out completely inactive when tested against CV-B5 and Sb-1, while compounds 16a,b-26a,b were active against CV-B5 and Sb-1 but inactive when tested against hRSV. In this series, as previously seen with benzamide derivative, the insertion of an aromatic moiety on the urea nitrogen leads to total loss of activity. The newly synthesised 4-F urea derivatives showed an additional anti-Sb-1 activity, while none of the previously tested compounds were found to be active.
| Compounds | Vero76 | CV-B5 | Sb-1 |
|---|---|---|---|
| - | aCC50 | bEC50 | - |
| 16c | >100 | 53 | >100 |
| 17c | >100 | 6.9 | 20.5 |
| 18c | >100 | 5.5 | 17.5 |
| 19c | >100 | >100 | >100 |
| 20c | 87 | >87 | >87 |
| 21c | >100 | >100 | >100 |
| 22c | >100 | >100 | >100 |
| 23c | >100 | >100 | >100 |
| 24c | >100 | >100 | >100 |
| 25c | >100 | >100 | >100 |
| 26c | >100 | >100 | >100 |
| Ref. compds | - | - | - |
| Pleconaril | 77±6.8 | 0.005±0.002 | 2±0.6 |
| Compounds | Vero76 | CV-B5 | Sb-1 | Ref |
|---|---|---|---|---|
| - | aCC50 | bEC50 | - | - |
| 16a | >100 | 10 | >100 | [17] |
| 16b | >100 | >100 | >100 | [17] |
| 16c | >100 | 53 | >100 | - |
| 16d | >100 | >100 | >100 | [16,25] |
| 17a | 100 | >100 | >100 | [17] |
| 17b | >100 | >100 | >100 | [17] |
| 17c | >100 | 6.9 | 20.5 | - |
| 17d | 30 | >30 | >30 | [16,25] |
| 18a | >100 | >100 | >100 | [17] |
| 18b | >100 | >100 | >100 | [17] |
| 18c | >100 | 5.5 | 17.5 | - |
| 18d | 90 | >95 | >95 | [16,25] |
| 19a | >100 | 16 | >100 | [17] |
| 19b | >100 | 53 | >100 | [17] |
| 19c | >100 | >100 | >100 | - |
| Ref. compds | - | - | - | - |
| Pleconaril | 77±6.8 | 0.005±0.002 | 2±0.6 | - |
The antiviral activity profile of these newly designed and synthesised compounds was compared with others that we previously reported, to extend the SARs analysis. In the series of N-(4-(R-2H-benzo[d] [1-3]triazol-2-yl)phenyl)alkylamides, the introduction of a fluorine atom in position 4, resulted in a power increase on both viral strains if compared to the parental compounds without any substituent on the benzotriazole scaffold or with methyl groups which were found only slightly active, while derivatives with two chlorine atoms were totally inactive (Table 3). Concerning the series of N-(4-(R-2H-benzo[d] [1-3]triazol-2-yl)phenyl)-4-aryl-benzamides, the introduction of a functionalized aromatic substituent was brought to a total loss of activity. The only exception was represented by already known derivatives with two chlorine atoms, which were active towards hRSV, CV-B5, BVDV and HNTV. 3-(4-(R-2H-benzo[d] [1-3]triazol-2-yl)phenyl)urea derivatives series showing a peculiar trend; only compounds with alkyl chains linked to urea nitrogen were proven active. New derivatives with fluorine atom in position 4 were detected as the best when compared with parental published compounds, both for the EC50 values and for the wider spectrum of activity, since they are also active towards Sb-1. The replacement with two chlorine atoms completely changed the activity spectrum as they were found inactive against enteroviruses, but very active when tested against hRSV and HTNV.
CONCLUSION
Presently, we can conclude that N-(4-(2H-benzo[d] [1-3] triazol-2-yl)phenyl-R-amide is a good chemical scaffold for the development of new antiviral molecules. To gain the best anti-enterovirus profile, the substituent on benzotriazole moiety should be small as a fluorine atom, and in position 4; the substitution on the amide nitrogen must be with an alkyl short chain. The replacement of the amide group with alkyl-urea groups was beneficial for the antiviral activity. On the contrary, in both series (amide derivatives and urea compounds), the presence of aromatic substituents caused a total loss of activity. According to the high selectivity, lack of cytotoxicity and interesting potency, we can select 7c, 17c and 18c as lead compounds. Future aims will be to extend the screen and to involve in our research additional enteroviruses with enormous medical impacts such as enterovirus A71 and enterovirus D68.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The study is financially supported by the “University of Sassari, Italy, Fondo di Ateneo per la ricerca 2019 Grant: FAR2019CORON”, and “Regione Autonoma della Sardegna, Italy, Grant: RASSR01499”, and the “MIUR (Italy), PRIN 2015, Grant: 2015C7PCYZ.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Authors acknowledge the generous financial support from the University of Sassari, Italy, Regione Autonoma della Sardegna, Italy, and MIUR (Ministero dell'Istruzione, dell'Università e della Ricerca), Italy.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers website along with the published article.


