REVIEW ARTICLE
Synthetic Methods of Medicinally Important Heterocycles-thiazines: A Review
Praveen Kumar Sharma1, *, Andleeb Amin1, M. Kumar2
Article Information
Identifiers and Pagination:
Year: 2020Volume: 14
First Page: 71
Last Page: 82
Publisher ID: TOMCJ-14-71
DOI: 10.2174/1874104502014010071
Article History:
Received Date: 03/05/2020Revision Received Date: 04/08/2020
Acceptance Date: 05/08/2020
Electronic publication date: 22/09/2020
Collection year: 2020

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Heterocyclic compounds containing N and S atoms have unique properties so that they can be used as potential reactive materials in pharmacokinetic systems. In medicinal chemistry, the therapeutic applications of nitrogen sulphur heterocycles are well known. Especially, Thiazines attract the attention of chemists due to their great bioactive behavior. The present study is a review of the work carried out by the research community for the synthesis of novel, effective, medicinally important heterocyclic compounds-thiazines.
1. INTRODUCTION
Studies of heterocycles, containing heteroatoms such as Sulphur and Nitrogen, are definitely one of the supreme targeted areas in heterocyclic chemistry. They are extensively used in several studies of natural products and pharmaceutical agent’s synthesis [1-3]. 1,4-Thiazine ring systems are considered a significant heterosystem in heterocyclic chemistry because it constitutes the skeleton of various natural products, such as conicaquinones A and B [4], the two cytotoxic terpene, quinones, xanthizone [5], and xanthiside [6] Fig. (1).
1, 4-Thiazine ring containing heterocycles is also known to play a vital role in pigments and dyestuffs [7, 8]. In addition to this, 4H-1,4-benzothiazines and their derivatives have been exposed to show wide ranging biological activities [9, 10] such as anticancer [11, 12], antifungal [13, 14], antagonistic [15], antioxidant [16], antihypertensive [17], analgesic [18], cardiovascular activity [19], antibacterial [20], antimalarial [21], antimicrobial [22], etc. Figs. (1-9).
The pharmaceutical/biological activities range of benzothiazines (4H-1,4-benzothiazines) has been recognized by the structural flexibility because of folding along N-S axis. The folding angle is affected to a larger degree by the arrangement of the substituents and nature. The basic features present in benzothiazines (4H-1,4-benzothiazines) hetero system are similar to that existing in 10H-phenothiazine, which marked them industrially as well as therapeutically interesting [23-48] (Figs. 10 and 11).
In the present time, human society comes in contact with different types of problems related to health and some of them are pandemic in nature. These problems arise in a short time period and are responsible for the loss of life on a large scale. In this type of situation, society put their eyes towards research communities , especially chemist, for providing solution either in terms of synthesis of new chemicals which can be used as potential medicinal material against said diseases, or production of existing chemical material in a short time period so that they never face the problem of shortage. Synthesis of new products is quite difficult and requires ample time for testing, clinical trials, and permissions from concerned authorities. Thus, chemists generally focus on the alternate synthetic methods of already tested chemical compounds. To overcome the challenge of synthesis time, researchers give more focus on the development of new methods through which the desired compound can be synthesized in a short time period without comprising on the environmental conditions. Thus, keeping this in mind, the main objective of the present article is to provide information to the research community regarding synthetic methods of most dominating heterocycles: thiazine ring systems.
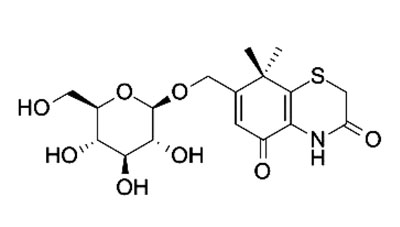 |
Fig. (1). Xanthiside. |
 |
Fig. (2). Vasorelaxant. |
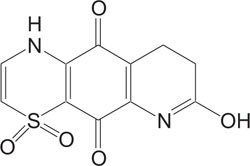 |
Fig. (3). Antimalarial agent. |
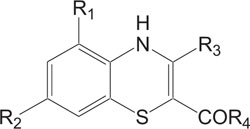 |
Fig. (4). Antibacterial agent. |
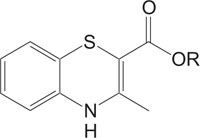 |
Fig. (5). Antagonistic. |
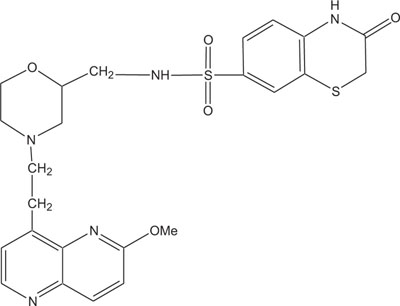 |
Fig. (6). Antibacterial against Neisseria gonorrhoea. |
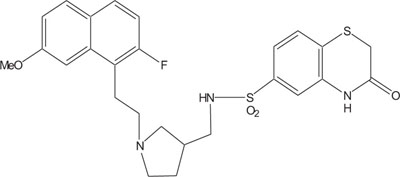 |
Fig. (7). Antimicrobial against Staphylococcus aureus. |
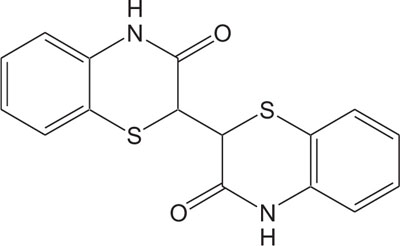 |
Fig. (8). Natural Pigment. |
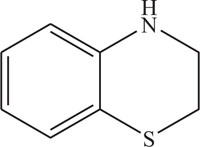 |
Fig. (9). Antiviral against zika virus. |
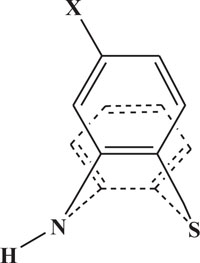 |
Fig. (10). Phenothiazine. |
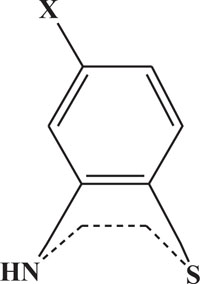 |
Fig. (11). 4H-1,4-Benzothiazine. |
2. REVIEW OF LITERATURE
In view of continuing interest of the synthetic chemists in therapeutically interesting 1,4-benzothiazine hetero system(diverse biological activities and desirable pharmacokinetics) discovery, a variety of synthetic methods incorporating the preparation of benzothiazine especially 4H-1,4-benzothiazine skeleton, have been developed. Some of the important methods available in the literature and which have been widely used for the synthesis of 4H-1,4-benzothiazines are presented as:
T. Yamada et al. have reported a convenient way for the synthesis of benzothiazines(4H-1,4-benzothiazines) by the reaction of 2-amino-benzenethiols(3.8g) with ethyl 2-chloroacetoacetate (5.0g) with refluxing in ethanol for 30 minutes and cooling. Reaction products were further added to water (120ml) (Scheme 1). The collected precipitate was crystallized in methanol with 52% yield. [49, 50].
To overcome the problems of low yield, excess use of hazardous solvents, and more use of energy in previous methods, different groups of renowned researchers including R.R. Gupta et al., S. Kano et al., G. Liso et al., further extended above approach which was used for the synthesis of substituted benzothiazine, especially 4H-1,4-benothiazines, by the reaction of 2-aminobenzenthiol and their derivatives with different reactants, such as cyclohexan-1,3-dione, cyno-thiomethylacetophenone, acetylenic nitriles, and esters, in the presence of most suitable agent DMSO [51-53] (Schemes 2-4).
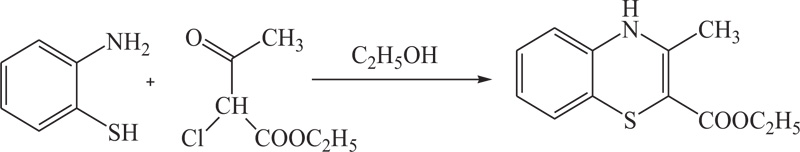 |
Scheme (1). Synthesis of Ethyl-3-methyl-4H-1,4-Benzothiazine. |
 |
Scheme (2). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with cyclohexan-1,3-dione. |
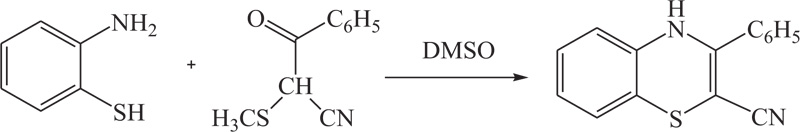 |
Scheme (3). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with cyno-thiomethylacetophenone. |
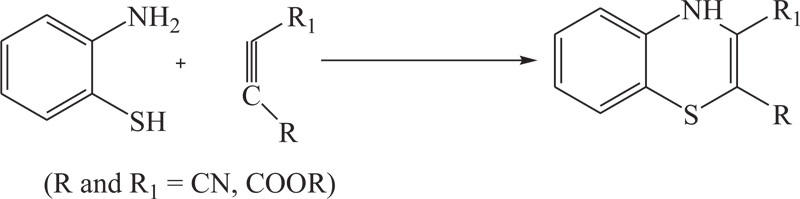 |
Scheme (4). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with acetylenic nitriles. |
In the same way, to increase the yield of product and reduce organic synthesis problem observed during the synthesis of most valuable nitrogen sulphur containing heterocycles, such as benzothiazines, A.Y. Chenko et al. and H. Nagase et al. used different approaches in which benzothiazines (4H-1,4-benzothiazines) have been prepared by the reaction of 2-aminobenzenethiol/sodium salt of N-substituted 2-aminobenzenethiols with bromoketones/phenacyl bromide [54,55] (Schemes 5-6).
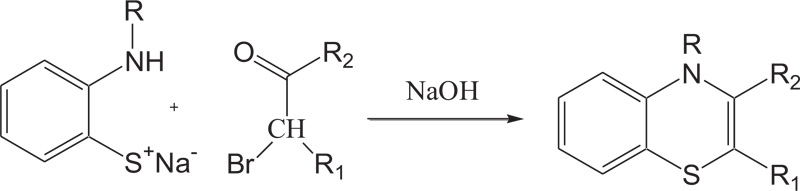 |
Scheme (5). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with bromo-ketones. |
 |
Scheme (6). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with phenacyl bromide. |
All the above methods are used for the synthesis of thiazine hetero systems. Due to organic synthesis complexity, regular use of hazardous chemicals, time-consumption, and lesser yield, these methods are phased out. To overcome all the above problems, Sheibani et al. have also reported the preparation of benzothiazines(4H-1,4-benzothiazines) by the following methods [56].
(a) The reaction of S-S dimer of 2-aminobenzenethiol with enaminones and esters in the presence of triethylamine provides 1,4-benzothiazines. In this reaction, nucleophilic addition of enaminones to disulphide of 2-aminobenzenethiol is followed by cyclization by Michael type addition of NH2 group to C=C bond to provide 1,4-benzothiazines (Schemes 7).
(b) Benzothiazines(4H-1,4- benzothiazines) have also been prepared by the reaction of alkylacetoacetates with disulphides in the presence of catalytic amounts of triethylamine (Schemes 8).
(c) The reaction of 2-aminobenzenthiols with β-diketones in the presence of H2O2/NaOH/H2O has also been reported for the preparation of 1,4-benzothiazines (Schemes 9).
(d) Sheibani et al. have also reported the reaction of acetylenic esters with 2-aminobenzenethiols for the preparation of 1,4-benzothiazines (Schemes 10).
V. Tandon et al. have stated ionic, liquid-assisted preparation of substituted 1,4-benzothiazines by the reaction of alkyl sulphanylamines with α-bromoacetylbromide. It was observed that the alkyl sulphanylamine with small alkyl groups are transformed into 1,4-benzothiazines easily with higher yields [57] (Schemes 11).
W. Zhang et al. have reported the synthesis of medicinally valuable benzothiazine (1, 4-benzothiazines) by the reaction of bis(o-nitrophenyl) disulphide with Kagan’s reagent [58] (Schemes 12).
M. Kumar et al. synthesized 1,4-benzothiazines incorporating chemical functionalities through a simple and appropriate regioselective synthesis involving the reaction of 2-aminobenzenthiols with diketone/ ketoester in the presence of dimethylsulfoxide. This method has been stated more suitable methods as previously reported methods used for the synthesis of 1,4-benzothiazines, as far as the yields of 1,4-benzothiazines are concerned [59-67] (Schemes 12-13).
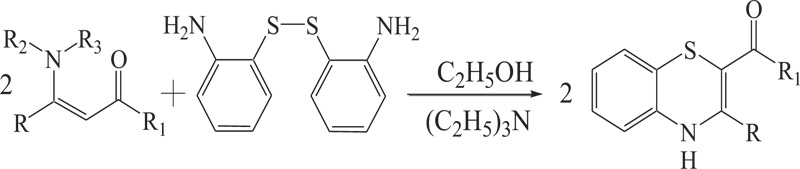 |
Scheme (7). Synthesis of 4H-1,4-Benzothiazine by nucleophilic addition of enaminones to disulphide of 2-aminobenzenethiol. |
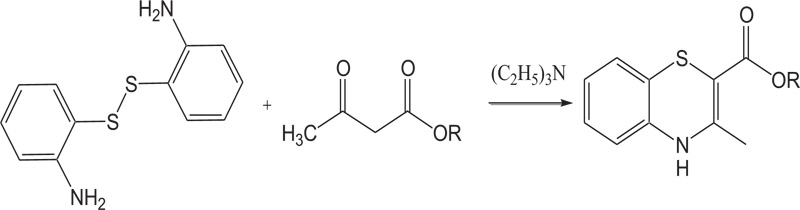 |
Scheme (8). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with alkylacetoacetates. |
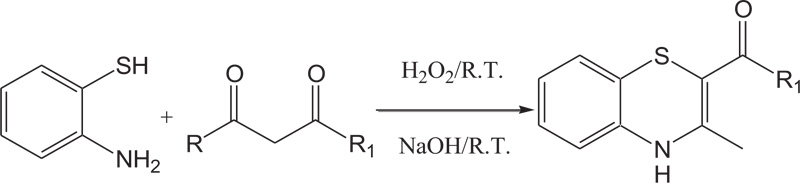 |
Scheme (9). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with β-diketones in the presence of H2O2/NaOH/H2O. |
 |
Scheme (10). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with acetylenic esters. |
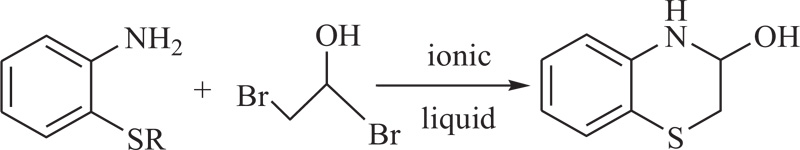 |
Scheme (11). Synthesis of 4H-1,4-Benzothiazine by the use of ionic liquids. |
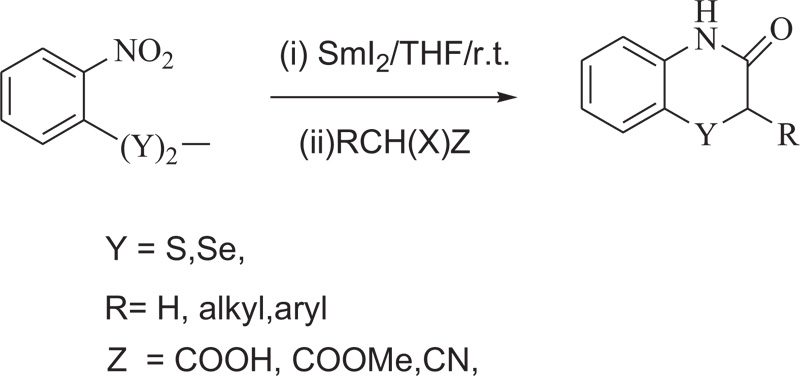 |
Scheme (12). Synthesis of 4H-1,4-Benzothiazine by the use of ionic liquids. |
 |
Scheme (13). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with beta-di-ketones. |
Mane et al. have extended this method to prepare 1,4-benzothiazines using hydrazine hydrate in the place of dimethyl sulfoxide under solvent-free conditions and reported that 2-aminobenzenthiol is oxidized by hydrazine, in the presence of air, into disulphide similarly as by dimethyl sulfoxide and undergoes cyclo condensation with diketones/ ketoesters involving in situ formation of enaminoketone as an intermediate [68] (Schemes 14).
Pratap et al. have designed a green, economical yet efficient synthesis of most biodynamical thiazine form:1,4-benzothiazines involving oxidative cycloaddition of 2-aminobenzothiol and 1,3-dicarbonyls in the presence of a cheaper biocatalyst baker’s yeast. Ultrasonication is also used to expedite the condensation process as it is responsible for the interference of the yeast cells for the fast discharge of the enzymes [69] (Schemes 15).
 |
Scheme (14). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with Phenyl hydrazine |
 |
Scheme (15). Synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol with beta-diketone in presence of Baker’s Yeast. |
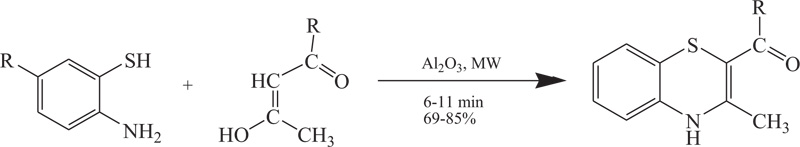 |
Scheme (16). Microwave assisted synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol. |
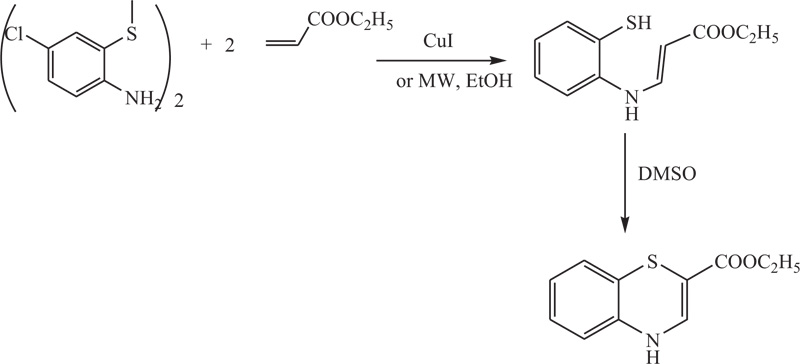 |
Scheme (17). Microwave-assisted synthesis of 4H-1,4-Benzothiazine by the reaction of 2-aminobenzenethiol derivatives. |
Paul et al. reported that aminobenzothiols and β-ketoester /β-diketones(using basic alumina as support under microwave irradiation) reacted with each other and produced medicinally important benzothiazines. The yield so obtained was found to vary in accordance with the amount of support taken and it was observed that maximum yields were obtained with 5-6 g of support versus 2mmol of the reagents [70] (Schemes 16).
Lizhi Fu et al. described a convenient method especially required in the synthesis of benzothiazines and their derivatives, which involves coupling between 2-amino-5-chlorophenyl disulphide and ethyl acetylenecarboxylate. The process is catalyzed using CuI or can be promoted via microwave irradiation, which expedites it and reduces the generation of by-products. Finally, the cyclization of the obtained compound with DMSO, followed by the oxidation using m-CPBA, afforded 4H-1,4-benzothiazines [71] (Schemes 17).
CONCLUSION
The overall aim of the review is to provide an idea, in detail, of novel methods used for the synthesis of medicinally important, biodynamic, and structurally diverse heterocycles. The present review article provides a platform for discovering important entities for pharmaceutical industries by new methods superior to the existing ones.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.








