All published articles of this journal are available on ScienceDirect.
Synthesis and Evaluation of New Derivatives of Busulfan as an Anti-carcinogenic Drug against k562 Cancer Cells Using the AO / PI Method
Abstract
Background:
Busulfan is a DNA alkylating drug used to treat chronic myeloid leukemia (CML). This type of leukemia affects bone marrow cells or myocytes, which make up bone marrow tissue having a chronic process. Leukemia is a progressive and malignant disease of the body's hematopoietic organs. The disease is caused by the underdevelopment and proliferation of white blood cells and their precursors in the blood and bone marrow.
Objective:
The synthesis of Busulfan drug and the conjugation of Busulfan from the anomeric position of acetylated galactose as a new derivative of Busulfan and its anti-cancer effect on K562 cancer cells are among the most important objectives of this study.
Methods:
In this work, galactose was acetylated with acetic anhydride in the presence of sodium acetate, then the anomeric position of acetylated carbohydrate deacetylated using imidazole to obtain the final product through the reaction of Busulfan. Anti-cancer activity of the product was evaluated against K562 cancer cells. The proliferation and survival of K562 cancer cells and PBMCs (peripheral blood mononuclear cells) using the AO/PI method were studied. Furthermore, 1- (2, 3, 4, 6-tetra-O-acetyl-Iβ-D-galactopyranosyl) -4- (methyl sulfonyl oxy) butane was evaluated against cancer cells as a new derivative.
Results:
In this study, the Acridine Orange / Propidium Iodide (AO/PI) method was performed using fluorescence microscopy to evaluate the efficacy of a synthesized product. The effective dose of 0.02 mg/L of 1- (2, 3, 4, 6-tetra-O-acetyl-Iβ-D-galactopyranosyl) -4- (methyl sulfonyl oxy) butane was observed to cause 96% of cancer cells necrosis.
Conclusion:
1- (2, 3, 4, 6-tetra-O-acetyl-Iβ-D-galactopyranosyl) -4- (methyl sulfonyl oxy) butane as a new derivative of Busulfan acts non-specifically in the cell cycle by inhibiting DNA activity by alkylation and binding to the DNA strand. Alkylating agents usually bind an alkyl group to the number seven nitrogen atom in the guanine nucleotide in a DNA molecule.
1. INTRODUCTION
Busulfan is an anti-cancer chemotherapy drug also available under the trade names Bosulfax and Miller. The drug is classified as an alkylating agent that binds an alkyl group to a DNA molecule, thus preventing DNA replication. Alkylating drugs are most active in the cell resting phase. Thus, they are cell cycle-nonspecific drugs. They are widely used in the treatment of chronic myelogenous leukemia as well as ovarian cancer. On the other hand, due to its alkylating properties, busulfan has a negative impact on the DNA of cells, having more dividing power. For this reason, it exerts the greatest effects on testicular spermatogonia. However, not all spermatogonia are killed by busulfan [1, 2]. Busulfan has many side effects, and they occur in more than 30% of patients, including a reduction in the number of blood cells, nausea, vomiting, diarrhea, loss of appetite, constipation, ulcers in the mouth and throat, headache, darkening of the skin, hair loss, damage to the testes, and fertilization disorders. This drug has not been an approved treatment method until the reduction of its side effects [3]. Anti-cancer drugs in chemotherapy exert toxic side effects, which may be due to their interaction with common drugs provided to the patient via systemic administration [4, 5]. As a result, the chemotherapy protocol, often at sub-optimal doses, requires drugs to minimize toxicity, reduce the suffering of patients, and prevent residual disease, regrowth of the tumor, and drug resistance. Thus, the goal is to improve cancer treatment and the specific action of drugs targeting the tumor [6, 7]. Several drugs have been developed to increase the selective targeting of the tumour cells. In this regard, aminoglycosides showed the primary advancement regarding their demand and utilisation as anticancer drugs. Monosaccharides increase tumor consumption compared to normal cells, resulting in increased intracellular uptake of tumor cells [8,9]. Activation and release of glycogen-mediated drugs to the target site can be accelerated by a glycoside attached to a specific antibody. A chemical glycosylation reaction involves the coupling of the sugar to a glycosyl acceptor that forms a glycoside. If the acceptor is a sugar, it will be an oligosaccharide product. Moreover, it also involves coupling the glycosyl donor to a glycosyl acceptor by the appropriate activator.
Carbohydrates have been used for medicinal purposes, such as heparin in anticoagulant drugs, antibiotics, vaccines, etc [10, 11]. However, carbohydrates and their role have not been explored much in drug development [12]. They have not been evaluated because of the complicated structures of oligosaccharides [13, 14]. As a result, there is a lack of convenient synthetic methods for synthesizing drug-bonded carbohydrates and analyzing these structures. Therefore, Busulfan drug coupling to carbohydrates is one of the most important reactions in the preparation of effective drugs. The acetylation of carbohydrates and coupling them with Busulfan for synthesizing new derivatives is a novel and emerging concept.
2. EXPERIMENTAL SECTION
All materials were obtained from Merck Company.1H NMR and 13C NMR spectra of products were measured using Brucker 400 AC spectrometer in CDCl3 or DMSO-d6 as a solvent at room temperature (University of Tabriz, Tabriz, Iran and Shahid-Beheshti University, Tehran, Iran).
2.1. Typical Procedure for Preparation of D-glucose Penta-acetate (2a)
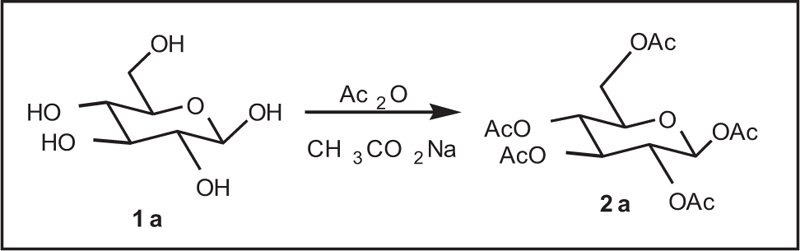
A mixture of D-glucose (15.0g), acetic anhydride (70mL), sodium acetate (15.0g), and butyl acetate (150mL) was refluxed with stirring for one-half hours. Then, the reaction mixture was added to water (100mL), and the mixture was stirred to obtain neutral solution with a 3% sodium hydroxide. After the concentration of the organic layer, it afforded 62.0g (yield 95%) of penta-acetyl-Iβ-D-glucopyranose as crude crystals. The crude crystals contained 13% of penta-acetyl-Iβ-D-glucopyranose, and after recrystallization in ethanol, 49.7g of pure penta-acetyl-Iβ-D-glucopyranose was achieved (m.p= 132AβC, yield 77%). Spectral analysis of 1H NMR: I' (ppm)6.6 (d, 1H, J = 3.6Hz), 5.80 (t, 1H, J = 9.8Hz), 5.33 (t, 1H, J= 9.8Hz,), 5.24 (dd, 1H, J = 10.5, 3.7Hz), 4.29 (dd, J = 12.2, 4.3Hz), 4.08-4.15 (m, 1H), 4.0 (dd, J = 12.3, 2.2Hz), 1.50-1.70 (3s, 5CH3).
3. MATERIALS AND METHODS
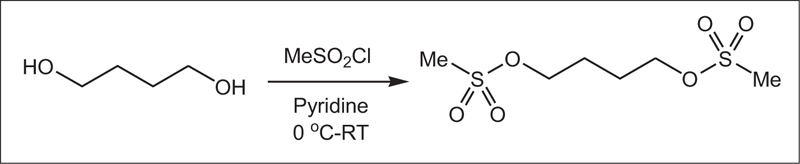
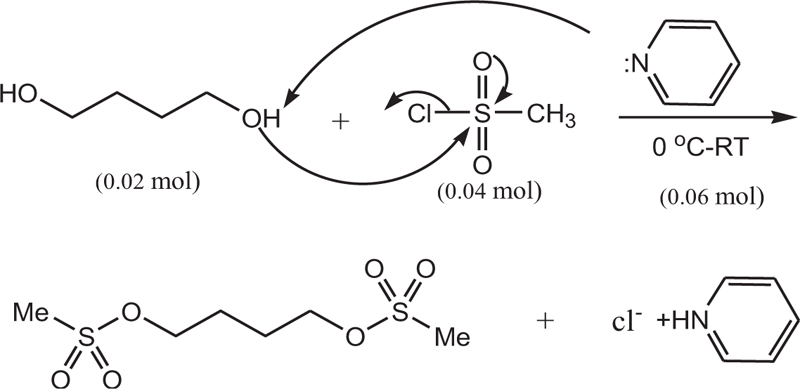
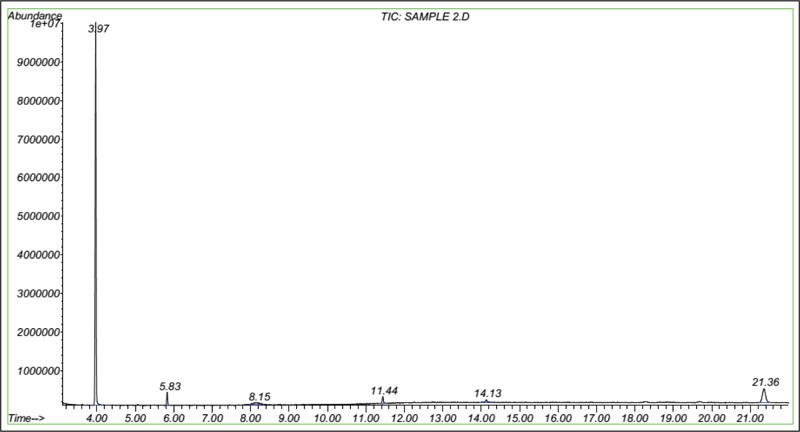
In a 50 ml flask, pyridine (0.06 mol, 4.83 ml) was poured, and in a water bath, the temperature of the mixture was reduced to zero. Then, 1,4-butanediol (0.02 mol, 1.76 mL) was added to the mixture and stirred for 30 min. Methane sulfonyl chloride (0.04 mol, 3.1 ml) was then added dropwise to the mixture. The resulting mixture was stirred at room temperature for 6 h. It was then refrigerated for 8 h. After this step, a few drops of water were added to neutralize the remaining methane sulfonyl chloride, followed by 5 ml of water. Furthermore, the precipitate was filtered and dissolved in ethyl acetate (20 ml) and washed with salt water (5 ml) and water (5 ml). Finally, solubilization was performed, and the resulting precipitate was crystallized by ethanol. The white crystalline precipitate was obtained with a melting point of 118 AβC and a weight of 3.5 g with an efficiency of 85%. Figs. (1-5) show the synthesis of busulfan.
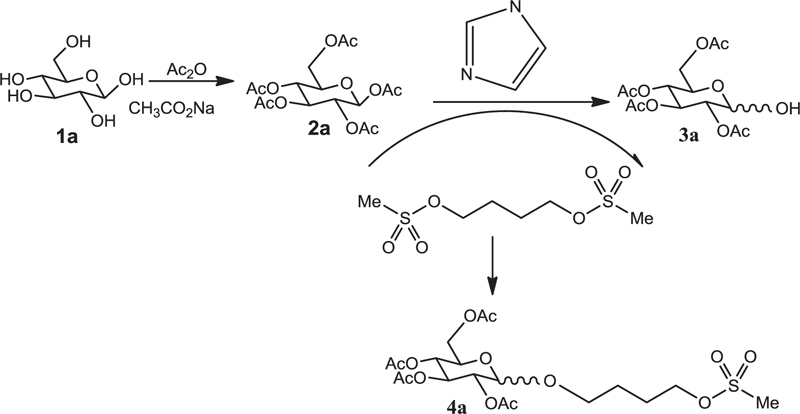
3.1. General Method of Coupling Busulfan to Glucose Penta-acetate
The glucose penta-acetate obtained from the previous step (1.5 mol, 0.55 g) was dissolved in pyridine (2mL), and the solution was cooled to 0AβC. Drops of mesyl-chloride (2 mmol, 0.155 ml) were added and stirred for 6 h. Distilled water was added (5 mL) to the reaction mixture until the formation of a precipitate. The precipitate was dissolved by CH2Cl2 (12 ml), and the organic phase was extracted with salt water (2 x 6 ml). It was then purified by dry sodium sulfate and finally purified using ethanol. Ft-IR (KBr, I... cm-1): 2958, 1753, 1371, 1232, 1059.
3.2. Investigation of Cell Viability
In this study, to evaluate the viability of cells with 4a compound, Acridine Orange / Propidium Iodide (AO / PI) method was performed using a fluorescence microscope. The viability of cells was expressed as a percentage. For this purpose, the sample was stained with AO/PI at a rate of 10 Aβ l / ml and incubated for a quarter at 37 Aβ C and 5% carbon dioxide. After incubation and washing twice with PBS solution, 10 I1/4L of propidium iodide was added and incubated for 5 minutes at 37 Aβ C and 5% carbon dioxide. In the last step, samples were again washed with PBS solution and centrifuged for 10 minutes at 1400 rpm to remove the supernatant. Then, 50 I1/4L was removed from the bottom of the cell. After placing the slide under a fluorescence microscope with a 40 lens, 5 microscopic fields were examined.
3.3. Evaluation of the Effect of a Resultant Substance (carbohydrate-bound busulfan) on the Proliferation and Survival rate of K562 Cancer Cells and PBMC Cells
In this study, the effects of the resultant substance on cancer cells and PBMC cells were investigated. Concentrations were shown in micrograms of substance X per mL. Cell proliferation was assessed by the MTT method using an Elisa reader.
Cell suspension with a concentration of 1A-105 was prepared from PBMC cells and K562. Cells under optimal growth conditions in 3 groups of 104 K562 cells and 105 PBMC cells were cultured separately in 96 house culture plates in RPMI 1640 medium containing 15% FBS with different concentrations of compound 4a. One group was considered a control, and the other groups were incubated in the presence of different concentrations of Royal X for 72 hours. A total of 25 I1/4L of 5 mg / mL MTT solution (3-4,5-dimethylthiazole 2-yl 2,5-diphenyltetrazolium bromide) in PBS was added to each well and incubated for 4 hours under similar conditions. During this time, living, proliferating cells with MTT formed formazan. A total of 100 I1/4L of DMSO was added to each well. The color intensity was determined at 492 nm. RPMI 1640 culture medium was also used as a blank.
4. RESULTS AND DISCUSSION
4.1. The Procedure of Synthesis of Busulfan and Acetylation of Glucose
The reaction of glucose with acetic anhydride in the presence of sodium acetate and pyridine was completed within 4 hours and produced glucose penta-acetate (Fig. 1).
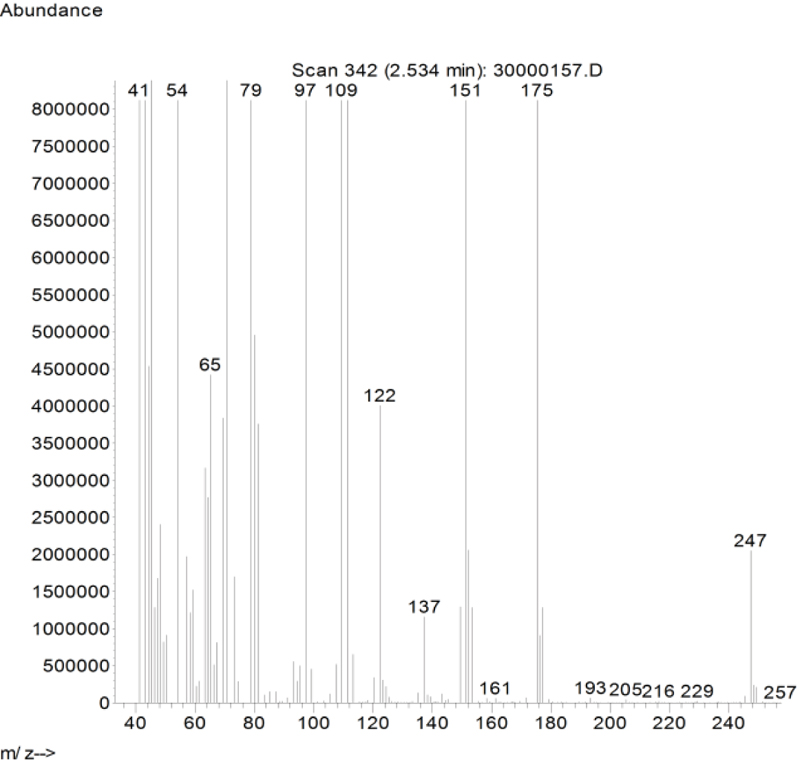
In this study, busulfan drug was designed and synthesized according to the general reaction (Fig. 2) and was identified by different spectroscopic methods, which were used for the interpretation of each spectrum. In the FT-IR spectrum, this compound eliminated the tensile vibration of the OH functional groups, confirming the formation of the drug compound. In the 1H NMR spectrum, a combination of three categories of peaks was observed. First (originally ternary) in the 1.92 ppm region corresponded to two methylene, second in the 4.29 ppm region corresponded to two methylene attached to oxygen atoms (originally ternary), and third in the 3.03 ppm region belonged to the methyl group. In the 13C NMR spectrum, this combination of three peaks was observed in the regions of 67.8, 36.4, and 24.4 ppm, confirming the formation of the desired composition. In the mass spectrum of this compound, the molecular ion peak of the compound at 247 m / z indicated the formation of the drug compound busulfan (Fig. 4). GC analysis diagram of this compound shows the Busulfan peak time of 3.9 min (Fig. 5).
The acetylated carbohydrate was dissolved into dichloromethane, and the reaction mixture was cooled at 5 AβC. Mesyl chloride (3 mL) was then added drop-wise and stirred for 12 h. Finally, by adding 10 mL of distilled water, a precipitate was formed and dissolved in DMF solvent. The organic phase was extracted and washed twice with distilled water and dried with sodium sulfate. The process of galactose acetylation and the release of anomeric position using imidazole and reaction with busulfan are shown in Fig. (6). Moreover, the processes of lactose acetylation and deacetylation of the anomeric position using imidazole and reaction with Busulfan are shown in Figs. (2 and 3), respectively.
The samples of carbohydrates, consisting of glucose, galactose, xylose, fructose and lactose, were acetylated using Ac2O/AcONa under 50-60AβC and anomeric configuration of acetylated carbohydrates using imidazole as a new catalyst was investigated. Busulfan coupling was performed at the anomeric position of sugar to prepare new drug derivatives. Their anti-carcinogenic properties were studied by AO / PI method (Fig. 6). In Ft-IR spectra, peaks at 1730 cm-1 showed a carbonyl group and peaks at 1000 cm-1 were related to the aliphatic group. Absorption peaks of esters appeared in the range of 1000-1300 cm-1 due to the acetylation of galactose while organizing carbonyl in position 2 showed the effect of the neighboring group, so the existing quitter group took apart from carbon no.1 and organized stable carbocation. In this mode, there was a possibility of attacking carbon anomeric by the nucleophilic agent. The absorption band at 1379 cm-1 was related to the symmetrical curvature of methyl CO-CH3, and absorption at 1221cm-1 was related to the neighbor C-O vibration of an ester carbonyl group. The absorption band at 1100 cm-1 was due to the ester tension of the alcoholic part of an ester.
4.2. Evaluation of Anti-Cancer Efficacity Using AO/PI Cell Viability Survival Method
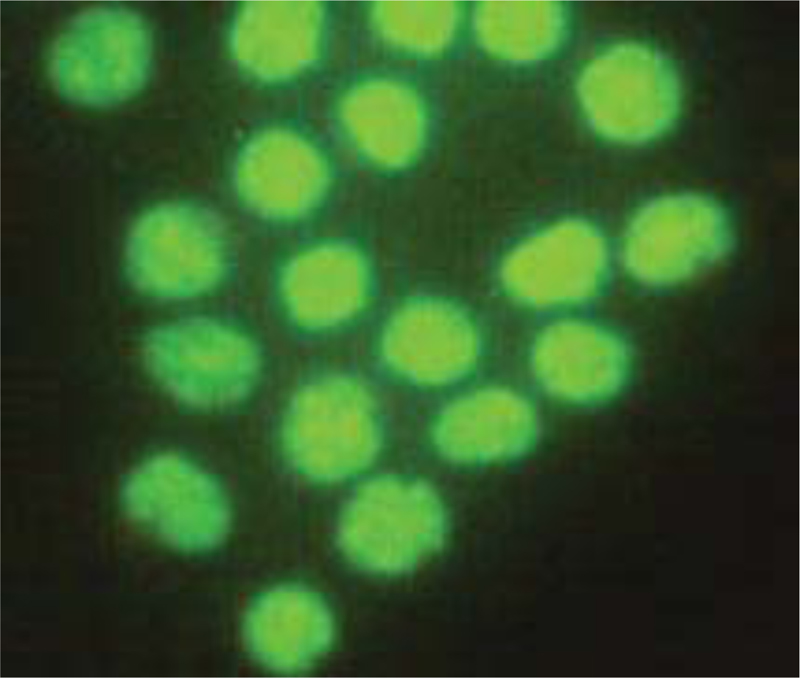
Microliters of cell suspension containing 100 cells were mixed with one microliter of staining solution and stirred slowly and thoroughly. Each sample was stained with color immediately before microscopic evaluation, and the samples were examined under a microscope, and the cells were counted. A microliter of the sample mixture was placed on a slide. At least 100 cells were counted with a fluorescein filter and a 60x object lens magnification. Depending on the cell type, different magnifications could be selected to distinguish the nucleus structure. Live and healthy cells would be uniformly green. Early apoptotic cells turn green and contain bright green spots in the nucleus due to chromatin condensation and nuclear fragmentation. Secondary (late-maturing) apoptotic cells absorb propidium iodide and are orange in color, except that, unlike necrotic cells, these cells often have dense fragmented nuclei. Orange-colored necrotic cells have a structure similar to healthy cells and do not have a dense nucleus. All cells that emit all or part of the orange light are considered dead, and all cells that emit only green light are considered alive.
4.3. Results of AO/PI Cell Viability Survival for Compound 4a
A total of 100 living cells were taken to determine primary apoptosis, delayed apoptosis, and necrosis. In the dual staining method of AO/PI, acridine penetrates all cells and causes the nucleus of cells to turn green. Propidium iodide only enters cells whose membranes are not healthy and causes a red color in their nuclei. So, green cells present healthy cells, whereas red cells present unhealthy cells. Cells that are light green with dense, fragmented chromatin are primary to apoptosis. Cells that are red with dense, fragmented chromatin are secondary to apoptosis, and those with normal, swollen, red nuclei are necrotic. Furthermore, the effective dose of the drug was determined to be 0.02 micrograms. The details of the cancer cell are given in Table 1 and Figs. (7-10).
| Entry | Chronic Myelogenous Leukemia | Property |
| 1 | Organism | Bone marrow |
| 2 | Morphology | Lymphoblast |
| 3 | Disease | Chronic myelogenous leukemia (CML) |
| 4 | Age | 45 years |
| 5 | Primary | NO |
| 6 | Gender | Female |
| 7 | Product Format | Frozen |
| 8 | Growth Mode | Suspension |
| 9 | Biosafety2 Level | 1 |
| 10 | Applications | Cell culture/growth conditions, stable cell transfection, transient transfection, gene expression, protein expression, protein purification |
| 11 | Karyotype | 2n = 46 |
| 12 | Storage Temperature | -1960C |


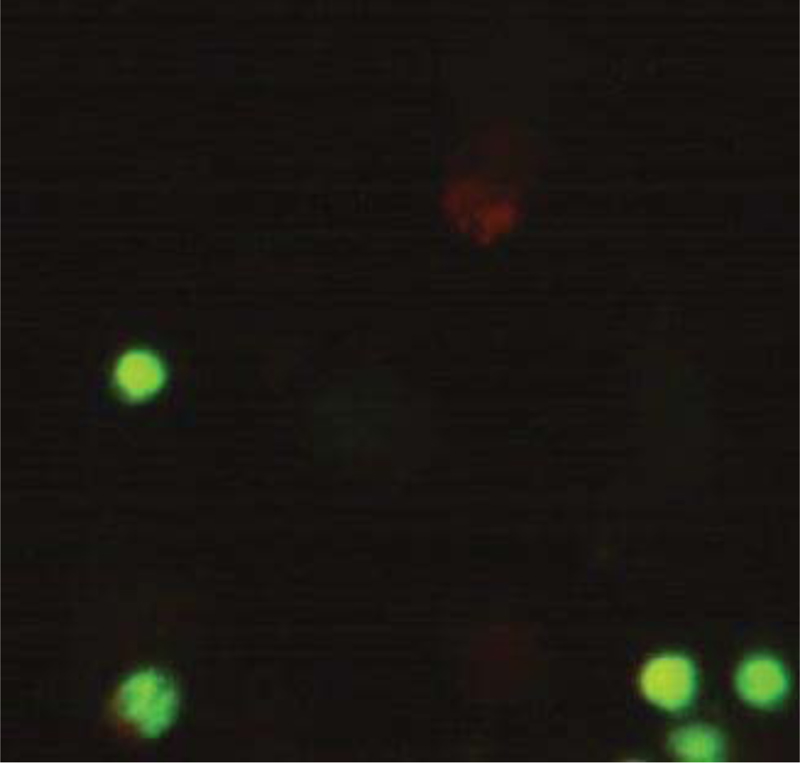
The K562 cell line derived from a CML patient was examined for characteristics of B and T lymphocytes and cell lines. K562 lacks the B markers of immunoglobulins, Epstein-Barr virus (EBV) genome and associated nuclear antigen, and receptors for EBV.
4.4. Cell Line Description
K562 cells were established as the first human immortalized myelogenous leukemia line. This cell line was derived from a 45-year-old female chronic myelogenous leukemia patient in blast crisis. They can be used as a highly sensitive target for the in vitro natural killer assay. Recently, studies have shown the K562 blasts are multipotential, hematopoietic malignant cells that spontaneously differentiate into recognizable progenitors of the granulocyte, erythrocyte, and monocytic series.
Service Guidelines: their facilities comply with biosafety regulations
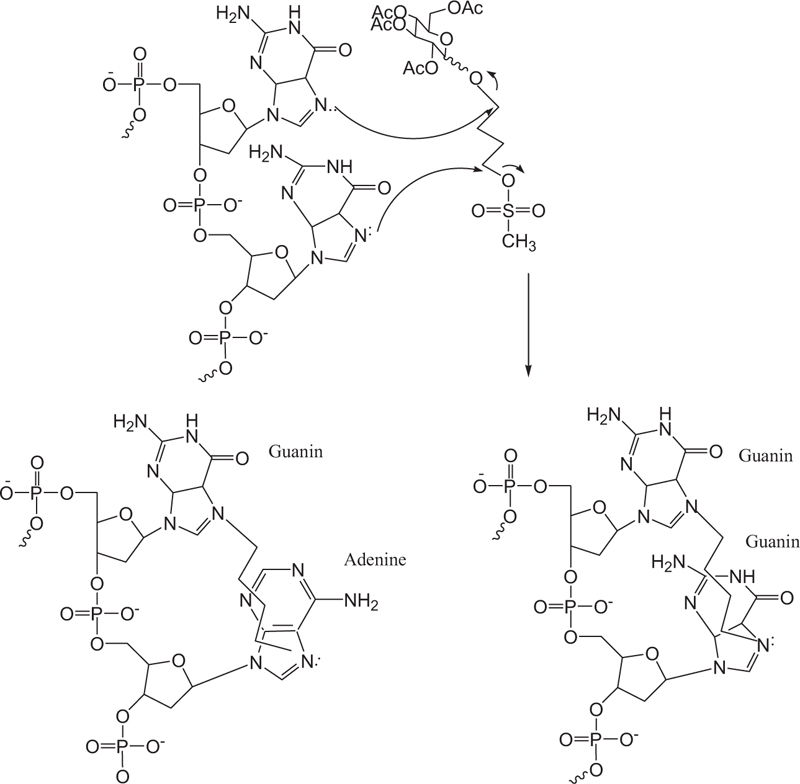
Busulfan acts non-specifically in the cell cycle by inhibiting DNA activity by alkylation and binding to the DNA strand. Alkylating agents usually bind an alkyl group to the number seven nitrogen atom in the guanine nucleotide in a DNA molecule (Fig. 11). A new derivative of busulfan di-alkylene agent binds to two different nitrogen atoms, thus causing cross-linking in DNA. DNA deformation inhibits tumor growth by preventing cell replication. The mechanism of action is through the alkylation of guanine adenine. Guanine-guanine cross-links occur through an SN2 reaction in which the guanine N7 nucleus attacks adjacent carbons to leave the mesylate group. This type of damage is not always repaired by cellular apparatus, thus causing cellular apoptosis.
CONCLUSION
In this study, anti-cancer efficiency was evaluated throughout AO/PI cell viability survival method, and results showed good to excellent efficiency of busulfan-bonded carbohydrates. Hence, our method could be regarded as a good protocol to increase anti-cancer activity of other drug-like compounds. The main advantage of our synthesis method is the high reaction efficiency (85%) and the simplicity of the reaction without the need for chromatographic methods. It should be noted that the high reaction efficiency and low cost of the process of separation and purification of the drug composition are important and significant economic factors for any process. Also, the synthesis of carbohydrate busulfan at the semi-industrial scale with high efficiency through this simple process can be performed successfully.
LIST OF ABBREVIATIONS
| DNA | = Deoxyribonucleic Acid |
| NMR | = Nuclear Magnetic Resonance |
| DMSO | = Dimethyl Sulfoxide |
| AO | = Acridine Orange |
| PBMCs | = Peripheral Blood Mononuclear Cells |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used in the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the research council of the Alborz University of Medical Science for supporting this study.
REFERENCES
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]
[PubMed Link]


