All published articles of this journal are available on ScienceDirect.
Phyto-Extracts and Silicone Gel (JUMI) in Reduction of Surgical Scar Post-joint Arthroplasty. A Randomized Control Trial
Abstract
Background and Objective:
To assess the clinical potency of JUMI Anti-Scar Cream (JASC) in the prevention of excessive scar formation post total hip replacement (THR) and total knee replacement (TKR).
Patients and Methods:
Our study was an open-label, prospective, randomized control to test the efficacy of JASC. After surgery, the same material was used in both groups to close the skin. After 14 days, once the staples were removed, JASC was applied twice a day for 60 days. Patients were seen at 6 and 12 weeks. At 3-month follow-ups, the scars were assessed using the Visual Analogue Scale (VAS) and Vancouver Scar Scale (VSS).
Results:
Forty patients were part of this trial; two patients in the control group were lost during follow-up. The average age-matched in both groups. There were no adverse or untoward effects due to the use of JASC. At 3 months, there were significant differences between the parameters assessed; VAS scores were P<0.0001. VSS parameters such as vascularity, pigmentation, pliability, and scar height were much lower compared to patients who had standard treatment (P<0.0001).
Conclusion:
Our study demonstrated that JASC was effective and successful in suppressing excessive scar formation. We believe that JASC has the potential as a prime anti-scar therapy, and we recommend more multicentric studies.
Clinical Trial Rg. No: This trial was registered with the Sri Lankan Trial Registry vide #2022/021.
1. INTRODUCTION
According to the American Academy of Dermatology, a scar appears as a natural step to repair wounded skin, and excessive scars result in social, emotional, and psychological effects [1]. Scar management costs in the US amount to $20 billion annually [2, 3]. Yearly, 250 million surgeries around the world cause scars-hypertrophic, keloid [4], and atrophic scarring as well as stretch marks (striae). Hypertrophic scars (HTSs) form from excessive collagen tissue and often regress spontaneously [5]. but they can continue to grow with an influx of fibroblast-derived proteins and collagen due to persistent inflammation and fibrosis [6]. Post-operative scarring is a reality in THR and TKR patients. These scars are cosmetically unappealing, and as the areas lack hair follicles and sweat glands, excessive scarring can even induce itching and pain. Scars develop even at the hands of skillful, experienced surgeons.
The current management of excessive scarring is empirical, unreliable, and capricious, with no established drug to prevent over-scarring. Bleomycin [7-10] is believed to inhibit collagen synthesis by influencing decreased stimulation via transforming growth factor-beta 1 (TGF-b1). Silicone is another agent that can decrease synthesis [11, 12]; silicone-based treatments have been reported to give better results over the last decade, but their operation mechanism is unclear. It has been suggested that silicone affects fibroblast activity via keratinocyte-mediated epidermal-dermal signaling pathways, decreasing volume while increasing elasticity in HTSs [13, 14]. In a randomized study, Kong et al. (2014) [15] found that silicone gel after TKR did not improve or inhibit pain and itching in the early postoperative period. Phytochemicals from medicinal plants have been utilized to cure many diseases, and plants are the primary source of over 25% of medicines [16]. Recently, the use of medicinal plants in the management of HTSs has been studied and found to be highly effective [17-22]. JASCTM is an optimum combination of silicone and phytochemicals effective in anti-scar therapy, but it has not stood the test of a clinical trial. This trial was done to determine the potency of JASC in individuals undergoing joint arthroplasty.
2. PATIENTS AND METHODS
Our study was an open-label, prospective, randomized control trial study carried out at King Fahd Hospital at a university in Al-Khobar, in which we tested the efficacy of JASC as an ultra-advanced formula (registered by AYUSH vide T 1826/Ayush/0051/2021/P/ Government of Telangana, India) with Phyto extracts (Centella asiatica extract, Curcuma longa, lavender oil, marshmallow, Musa paradisiaca, pineapple extract, and tea tree oil) and silicone gel. The study was intended to measure the reduction of surgical scars after TKA and THR and to compare results with those of patients who had standard wound and scar care, which included a timely review of the wound, appropriate cleansing and dressing, and early recognition and active treatment of wound complications. The study was approved by the Institutional Review Board of Imam Abdulrahman Bin Faisal University, Dammam, and the trial was registered with the Sri Lankan Trial Registry vide #2022/021. Participants gave informed written consent to participate and for their anonymized information, data, and clinical pictures to be published and presented. The inclusion criteria included all patients who signed the consent and were undergoing TKA and THR for primary osteoarthritis and avascular necrosis of the femoral head (ANFH); those who suffered from uncontrolled diabetes mellitus, neurovascular diseases, or any other immunological diseases were excluded from the study. Alternate patients were assigned to the study and control groups. After the procedures, wounds were closed in layers.
The subcutaneous layer was closed using 2/0 vicryl and skin with Covidiean Appose Single-Use Skin Stapler (Minneapolis, MN, United States). The staples were removed after 2 weeks, once the wounds had healed. All patients had a standard dressing and a knee brace after TKR. Twenty-four hours after surgery, patients began full weight-bearing walking with a walker and working on active assisted ROM exercises. Skin staples were routinely removed on day 14 after surgeries, and Primapore dressing was applied. Clinical photographs were taken before and after the study period using the same camera and filters; JASC was applied twice a day for 12 weeks. Patients were seen at 6 weeks and 12 weeks (earlier if needed). On follow-up at 6 and 12 weeks, scars were assessed by two independent surgeons (a specialist and a consultant who were not part of the study) using the VAS and VSS [23]. Side effects were monitored throughout the trial. At each scheduled visit, an independent surgeon who was not part of the research team conducted a safety assessment. If a patient decided to discontinue, the reasons for discontinuation were recorded.
Fig. (1) shows the Consort reporting of Trials.
2.1. Statistical Analysis
Mean scores for all parameters of the VS and VSS were calculated and expressed as means ±SD. A P value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS Version 25, OS X, for MacBook, document 428047.
| Parameters | JASC Group | Standard Treatment Group | P Value |
|---|---|---|---|
| Number of patients | 20 | 18 | - |
| Age (Years) | 57.85±12.9 | 56.3±11.2 | 0.6 |
| Male | 6 | 7 | - |
| Female | 14 | 11 | - |
| THR | 5 | 4 | - |
| TKR | 15 | 14 | - |
| VAS after removal of clips | 1.25±0.44 | 1.27±0.5 | 0.8 |
| Vascularity (0-3) | 1.2±0.4 | 1.35±0.5 | 0.5 |
| Pigmentation (0-2) | 2.15±0.36 | 2.2±0.4 | 0.68 |
| Pliability t (0-5) | 1.4±0.5 | 2.17±1.2 | 0.012 |
| Height (0-3) | 2.1±0.3 | 2.44±0.5 | 0.014 |
| VAS after 3 months | 0.5 | 1.27±0.5 | 0.0001 |
| Vascularity after 3 months (0-3) | 0.1±0.3 | 1.44±0.51 | 0.0001 |
| Pigmentation after 3 months (0-2) | 0 | 2.33±0.5 | 0.0001 |
| Pliability after 3 months (0-5) | 0.15±0.6 | 2.8±1.2 | 0.0001 |
| Height after 3 months (0-3) | 0.05±0.3 | 2.55±0.5 | 0.0001 |
3. RESULTS
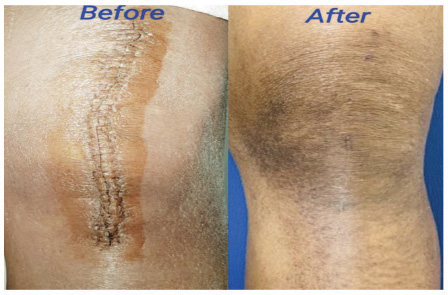
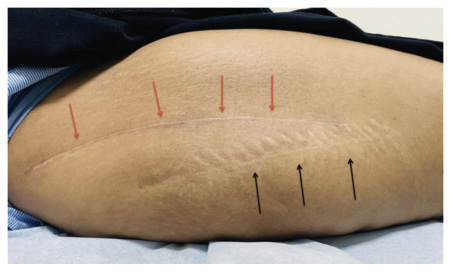
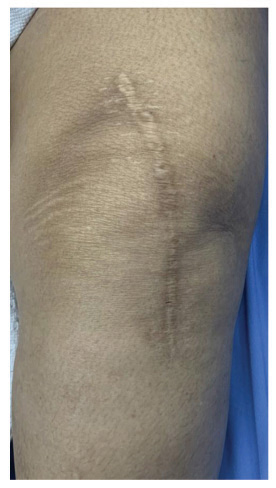
Forty patients were included in the study, but we lost two patients from the control group during follow-up. There were no adverse or untoward effects due to the use of the study cream. The groups were matched in age, sex, and type of surgery. The average age was 57.85±12.9 years in the study group and 56.3±11.2 years in the standard treatment group. The demographic data and the VAS and VSS evaluations are given in Table 1. At baseline, there was no difference between the parameters assessed; at 3 months, there were significant differences between the two groups’ VAS scores P<0.0001). VSS parameters such as vascularity, pigmentation, pliability, and scar height were much lower compared to patients in the control group (P<0.0001). (Figs. 1 and 2) describe patients who received JASC, while (Fig. 3) describes the control group, which received standard treatment.

4. DISCUSSION
Using JASC on postoperative scars showed colossal suppression of excessive scar formation; in some patients, scars were invisible after 60 days of use. All the parameters tested by the VAS and VSS were reduced significantly, becoming inconspicuous compared to the baseline and the patients who did not use JASC. In patients with standard postoperative treatment, the scars were highly visible, with prominent height and enhanced pigmentation.
Hypertrophic and abnormal scars cause immense discontentment as well as psychological and emotional difficulties, particularly if scars occur on a visible body surface. Patients have long sought medical help to reduce scar tissue, and hundreds of treatments have been tried with little success. Even with robust development and clinical research, many therapeutic approaches remain unsatisfactory. According to a recent extensive review by Khansa et al. (2016) [24], over 3835 pediatric and adult patients with all types of scars in 58 studies used 25 types of medications, with a few studies using treatments in combination but without satisfactory control of scar hypertrophy. One of the substances that did have some effect was a silicone-based treatment. It is suggested that silicone-based products affect fibroblast activity via keratinocyte-mediated epidermal-dermal signaling pathways, decreasing volume and increasing elasticity in HTSs [12-14].
The current belief is that a single agent may not be enough to reduce the size of a scar. Pressure therapy (PT) and silicone therapy are recommended as first-line noninvasive treatments for HTSs, but the effectiveness of this combination is not universally satisfactory, nor has its efficacy been confirmed through a randomized clinical trial.
Phytochemicals from medicinal plants have been utilized for the cure of many diseases, and plants are the primary source of over 25% of medicines [16]. Recently, the use of medicinal plants in the treatment of HTSs has been studied and found to be highly effective [18, 25, 26]. Phytochemicals have good anti-scar efficacy with no or few side effects in comparison to some chemical drugs, which can be effective but with side effects that outweigh the benefits. Phytochemicals such as asiaticoside [27], genistein [22], oleanolic acid, [28,] and resveratrol [29] are quite effective as anti-scar agents, with fewer side effects. Centella asiatica extract, an important phytochemical used in JASC, contains bioactive constituents such as triterpenoid saponins, flavonoids, phenolic acids, triterpenic steroids, and amino acids that improve skin health by increasing hydration and decreasing transepidermal water loss with anti-inflammatory effects. These actions keep scars healthy and restrict hypertrophy [30-32]. On the other hand, Musa paradisiaca is a strong antioxidant and affects various stages of wound healing. It influences wound contraction, increases wound-breaking strength and reduces the scar area, and has a good safety profile [33].
JASC is an optimum combination of silicone and seven other phytochemicals, which has now been tested in a randomized clinical study and has shown to be very efficacious and devoid of adverse reactions.
This study has limitations, as we studied only postoperative scars, and the follow-up period was only 3 months. There was no blinding of the investigators; more significance was given to the comparative clinical pictures. We did compare the study group with a group that received standard treatment, which could be accepted as a control group.
CONCLUSION
In conclusion, our study demonstrated that JASC was effective and successful in suppressing excessive postoperative scar formation and preventing unsightly over-scarring of surgical wounds. We believe scar prevention is preferable to managing an undesirable scar later, requiring immense cost, suffering, and psychological repercussions. As the literature suggests, no currently available scar therapy is globally successful, but we have reasons to believe that JASC has the potential as a prime anti-scar therapy.
LIST OF ABBREVIATIONS
| (JASC) | = JUMI Anti-scar Cream |
| (TKR) | = Total Knee Replacement |
| (VSS) | = Vancouver Scar Scale |
AUTHORS CONTRIBUTIONS
All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript;
Concept and Design: MSA, SAM, ASO
Operated the Patients: MSA, SAM, FMA
Follow Up: KWT, MMA, FMA
Data Analysis/Interpretation: MSA, MMA, FMA
Critical Revision of Manuscript: MSA, SAM, ASO
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by Imam Abdul Rahman Bin Faisal University, Dammam, Saudi Arabia, and registered with the Sri Lankan Trial Registry.
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. All the human were used as per the Helsinki Declaration guideline.
CONSENT FOR PUBLICATION
Informed written consent was obtained from all patients before the patients were included in the study. Patients gave consent that the anonymized information of their data and clinical pictures could be published and presented.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data of current study are available from author, [M.S.A]. on a reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


