Novel Benzylamine Derivatives: Synthesis, Anti-Mycobacterium Tuberculosis Evaluation and Predicted ADMET Properties
Abstract
Background:
Tuberculosis (TB), a disease caused by the bacillus bacteria Mycobacterium tuberculosis is one of the major contributors of ill health in the world. TB is ranked in the top 10 causes of death globally and it is the leading killer associated with a single infectious agent. According to the World Health Organization (WHO), global number of deaths associated with TB have been slowly declining with 1.3 million in reported 2016 and 2017, and 1.2 million reported in 2018 and 2019.
Objective:
The synthesis, characterisation, biological evaluations, and the prediction of ADMET properties of the novel benzylamine derivatives.
Methods:
Commercially available reagents and solvents were purchased from Sigma Aldrich and Merck (South Africa). All chemicals were used as received, unless otherwise stated. The synthesised crude compounds were purified by flash silica gel column chromatography (5 – 30% ethyl acetate in hexane). The successful formation and purity of the synthesised compounds was confirmed by NMR, HRMS and melting point.
Results:
The respective organic compounds were synthesised by treating 3-ethoxysalcyladehyde, 5-bromo-3-ethoxysalcyladehyde, 5-chloro-3-ethoxysalcyladehyde with various aromatic amines and the products were obtained in good to excellent yields. The 1H and 13C NMR spectra of all the products showed the appearance of the methylene signals ranging from 3.88 – 4.68 ppm and 42.25 – 52.57 ppm respectively. Additionally, most compounds showed anti-Mycobacterium tuberculosis activity that ranged between 20 and 28 µM.
Conclusion:
A total of 36 compounds were synthesised and successfully biologically evaluated against Mycobacterium tuberculosis (Mtb) H37RV strain. All compounds showed activity against Mtb at concentrations of > 20 µM < 28 µM with the exception of compound one that was active against Mtb at higher concentration (MIC90 > 125 µM).
1. INTRODUCTION
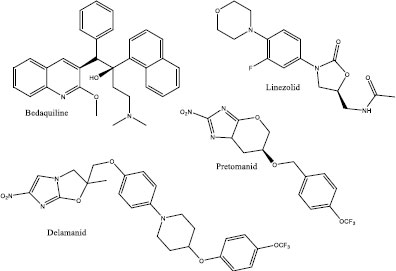
Tuberculosis (TB), a disease caused by the bacillus bacteria Mycobacterium tuberculosis is one of the major contributors to ill health in the world. TB is the top 10 causes of death globally and it is the leading killer associated with a single infectious agent. According to the World Health Organization (WHO), the global number of deaths associated with TB has been slowly declining with 1.3 million in reported 2016 and 2017, and 1.2 million reported in 2018 and 2019 [1-4]. Unfortunately, WHO also reported that the number of people infected with TB has increased from 6.4 million in 2017 to 7.1 million in 2019 [4]. Although significant progress has been achieved towards the development of antitubercular drugs, the emergence of multidrug-resistance tuberculosis (MD-R TB) and extensively drug-resistance tuberculosis (XDR-TB) coupled with the time required to completely cure tuberculosis (3 – 9 months) has compromised the management of tuberculosis. This compromise has led to the inefficient use of the first-line TB drugs (isoniazid, rifampicin, pyrazinamide, ethambutol and streptomycin) mainly due to the development of resistance towards these drugs [5-11]. To solve the resistance towards first-line TB drugs, intensive research efforts were directed at finding efficient and better-acting TB drugs. These efforts led to the development of antitubercular drugs such as bedaquiline [12], linezolid [13], delamanid [14] and pretonamid [15] (Fig. 1) as drugs that were aimed at treating MDR-TB and XDR-TB. Despite the successful introduction of these drugs to treat TB, there are still more challenges that still need to be addressed. Some of these challenges include treatment of people with HIV co-infection [16], shortened treatment time and drug costs [17], complicated regimen (number of pills required for the successful treatment) [18], effective and early TB detection [19], suitability of the current drugs for children (pill vs syrup) [20] and the emergence of totally-drug resistance TB (TDR-TB) strain [21]. Therefore, solving some or all these challenges will help WHO in meeting its goals of significantly reducing the burden of TB and subsequently, the reduction of the death toll associated with TB by 90% in 2030 [4]. More intensified research efforts are underway to identify novel methods and drugs for the treatment of TB. For example, studies of high doses of rifapentine as a replacement for rifampin for the shortened treatment time [22] while nanoparticles-based treatment is being explored for directed treatment [23, 24].
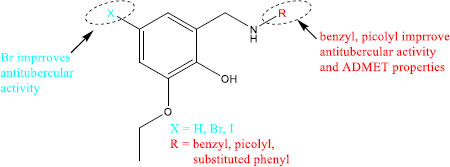
Benzylamine-containing compounds have been studied as potential drugs for treating an array of diseases including TB [25]. These investigations include their use as Kallikrein 5 inhibitors [26], Toll-Like Receptor 2 inhibitors [27], antifungal and antibacterial agents [28], possible anticancer agents [29, 30] and antidiabetic agents [31]. In addition to the above-mentioned biological properties, several drugs containing benzylamine have been approved for different ailments. For example, Levocetirizine is used for the treatment of hay fever, Clopidogrel is used as an antiplatelet drug to reduce the risks of heart disease and stroke, Donepezil is used for the treatment of Alzheimer's disease while Mirtazapine is used for the treatment of depression (Fig. 2) [32]. Taking advantage of the remarkable biological properties of benzylamine-containing compounds, here, we report the synthesis of benzylamine derivatives as potential antituberculosis agents. Halogens (iodine and bromine) were introduced on position 5 of the benzylamine to investigate their effect on the properties of the synthesised compounds. The benzylamine derivatives were biologically evaluated (in vitro) against Mtb H37RV strain while their cytotoxicity was on the Chinese Hamster ovarian (CHO) cell line.
2. EXPERIMENTAL PROCEDURES
2.1. General Information
Commercially available reagents and solvents were purchased from Sigma Aldrich and Merck (South Africa). All chemicals were used as received unless otherwise stated. The structural properties of the compounds were recorded and confirmed by: High-resolution mass spectra were recorded using Sciex X500R QTOF at the University of Limpopo Mass Spectrometry Facility; Melting points were obtained using Lasec/SA-melting point apparatus from Lasec company, SA (Johannesburg, South Africa); IR spectra were recorded using Bruker technologies Alpha Platinum ATR FTIR spectrometer; and Nuclear Magnetic Resonance (NMR) (Bruker Ascend 400 MHz Topspin 3.2); 1H NMR and 13C NMR spectra were referenced internally using solvent signals, 1H NMR: 7.250 ppm for CDCl3, 2.500 ppm for DMSO-d6; 13C NMR: 77.00 ppm for CDCl3, 39.40 ppm for DMSO-d6, respectively which were used as the solvents at room temperature. Chemical shifts are expressed in δ-values parts per million (ppm) and the coupling constants (J) in Hertz (Hz). Multiplicity of the signals is given as follows: brs = broad singlet, s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublet and m = multiplet.
2.1.1. Synthesis of 5-bromo-3-ethoxy-2-hydroxybenzaldehyde 239
Mixture of 2-hydroxy-3ethoxybenzaldehyde (1.500 g, 9.03 mmol) and N-bromo-succinimide (1.606 g, 9.03 mmol, 1 eq.) in acetonitrile (100 mL) for 18 hours. Subsequently, the reaction mixture was quenched with a saturated aqueous solution of ammonium chloride and extracted with ethyl acetate (3 x 30 mL). The combined organic fractions were dried with anhydrous sodium sulphate, solvent was removed on rotary evaporator and the resulting product was purified by flash silica gel column chromatography (5 – 30% ethyl acetate in hexane) to afford 5-bromo-3-ethoxy-2-hydroxybenzaldehyde 2 as a yellowish solid, 2.133 g, 97%. 1H NMR (400 MHz, CDCl3) δ 10.96 (s, 1H), 9.84 (s, 1H), 7.29 (d, J = 2.2 Hz, 1H), 7.16 (d, J = 2.1 Hz, 1H), 4.10 (q, J = 7.0 Hz, 2H), 1.49 (t, J = 7.0 Hz, 3H).40 HRMS (ESI) [M+H+]:m/z 244.1366; Calculated mass for C9H9BrO3 is 243.970.
2.1.2. Synthesis of 3-ethoxy-2-hydroxy-5-iodobenzaldehyde 3 39
A mixture of 2-hydroxy-3ethoxybenzaldehyde (1.500 g, 9.03 mmol) and N-iodosuccinimide (2.032 g, 9.03 mmol, 1 eq.) was added in acetonitrile (100 mL) for 18 hours. Subsequently, the reaction mixture was quenched with a saturated aqueous solution of ammonium chloride and extracted with ethyl acetate (3 x 30 mL). The combined organic fractions were dried with anhydrous sodium sulphate and solvent was removed on a rotary evaporator and the resulting product was purified by flash silica gel column chromatography (5 – 30% ethyl acetate in hexane) to afford 3-ethoxy-2-hydroxy-5-iodobenzaldehyde 3 as a bright yellow solid, 2.591 g, 98%, mp 94-96 °C. 1H NMR (400 MHz, CDCl3) δ 10.98 (s, 1H), 9.83 (s, 1H), 7.47 (d, J = 1.9 Hz, 1H), 7.29 (d, J = 1.8 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 1.48 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 195.21, 151.68, 148.54, 132.51, 127.19, 122.22, 79.80, 65.15, 14.59. HRMS (ESI) [M+H+]:m/z 292.978; Calculated mass for C9H9IO3 is 291.960.
2.1.3. General Synthetic Method for the Reductive-amination of 2-ethoxybenzaldehyde Derivatives
Mixture of 2-hydroxy-3-ethoxybenzaldehyde 1 (0.100 g, 0.602 mmol) or 5-bromo-3-ethoxy-2-hydroxybenzaldehyde 2 (0.100 g, 0.408 mmol) or 3-ethoxy-2-hydroxy-5-iodobenzaldehyde 3 (0.100 g, 0.342 mmol) and appropriate amine (0.632 mol, 1.05 eq.) in methanol (15 mL) was stirred for 18 hours before being reduced with sodium borohydride (0,04556 g, 1.20 mmol, 2 eq.) and stirred at room temperature for a further 4 hours. Subsequently, the reaction mixture was quenched with a saturated aqueous solution of ammonium chloride and extracted with ethyl acetate (3 x 30 mL). The combined organic fractions were dried with anhydrous sodium sulphate, the solvent was removed on a rotary evaporator and the resulting product was purified by flash silica gel column chromatography (5 – 30% ethyl acetate in hexane) to afford the appropriate product.
2.1.4. 3-Ethoxy-2-hydroxy-N-(phenyl)benzylamine (1a)
As a yellow-brown oil, 0.1324 g, 84%. 1H NMR (400 MHz, CDCl3) δ 7.23 – 7.13 (m, 2H), 6.92 – 6.86 (m, 1H), 6.79 (d, J = 4.8 Hz, 2H), 6.76 (dd, J = 7.3, 0.9 Hz, 1H), 6.72 (dd, J = 8.5, 0.9 Hz, 2H), 4.38 (s, 2H), 4.13 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.73, 145.85, 144.02, 129.15, 124.38, 121.06, 119.49, 118.18, 113.77, 110.75, 64.47, 44.09, 14.89. 3417, 2978, 1460, 1253, 1053, 747, 600cm-1. HRMS (ESI) [M+H+]:m/z 243.1366; Calculated mass for C15H17NO2 is 242.130.
2.1.5. 5-Bromo-3-ethoxy-2-hydroxy-N-(phenyl)benzylamine
(2a) As a light orange solid crystals, 0.1030 g, 78%, mp 88-90 °C. 1H NMR (400 MHz, CDCl3) δ 7.17 (dd, J = 8.4, 7.5 Hz, 2H), 7.02 (d, J = 2.1 Hz, 1H), 6.88 (d, J = 2.1 Hz, 1H), 6.75 (t, J = 7.3 Hz, 1H), 6.68 (d, J = 7.7 Hz, 2H), 4.33 (s, 2H), 4.07 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.60, 146.53, 143.11, 129.22, 126.24, 123.35, 118.32, 113.97, 113.62, 111.32, 64.86, 43.68, 14.74. HRMS (ESI) [M+H+]:m/z 322.0440; Calculated mass for C15H16BrNO2 is 321.040.
2.1.6. 3-Ethoxy-2-hydroxy-5-iodo-N-(phenyl)benzylamine(3a)
As a light brown solid, 0.1019 g, 81%, mp 78-81 °C. 1H NMR (400 MHz, CDCl3) δ 7.19 (ddd, J = 8.5, 5.5, 1.8 Hz, 3H), 7.04 (d, J = 1.9 Hz, 1H), 6.76 (t, J = 7.3 Hz, 1H), 6.71 – 6.66 (m, 2H), 4.31 (s, 2H), 4.05 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.61, 146.66, 143.99, 129.53, 129.18, 126.81, 119.58, 118.26, 113.59, 80.80, 64.80, 43.51, 14.73. Vmax (FTIR) 2968, 1488, 1273, 1078, 839, 772, 695, 576, 511 cm-1. HRMS (ESI) [M+H+]:m/z 370.0337; Calculated mass for C15H16INO2 is 369.020.
2.1.7. 3-Ethoxy-2-hydroxy-N-(benzyl)benzylamine (1b)
As a cream white solid, 0.1324 g, 88%, mp 76 °C. 1H NMR (400 MHz, CDCl3) δ 7.45 – 7.16 (m, 5H), 6.85 (dd, J = 8.1, 1.3 Hz, 1H), 6.79 – 6.73 (m, 1H), 6.68 – 6.63 (m, 1H), 4.12 (q, J = 7.0 Hz, 2H), 4.02 (s, 2H), 3.85 (s, 2H), 1.51 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.30, 147.22, 138.23, 128.55, 128.35, 127.45, 122.68, 120.53, 118.64, 112.12, 64.10, 52.51, 51.33, 14.89. Vmax (FTIR) 3294, 2958, 1470, 1275, 1043, 836, 774, 734, 699, 605, 554, 481, 432 cm-1. HRMS (ESI) [M+H+]:m/z 258.1482; Calculated mass for C16H19NO2 is 257.140.
2.1.8. 5-Bromo-3-ethoxy-2-hydroxy-N-(benzyl)benzylamine (2b)
As a brown solid, 0.1198 g, 87%, mp 87-89 °C. 1H NMR (400 MHz, CDCl3) δ 7.35 – 7.19 (m, 7H), 6.85 (d, J = 2.0 Hz, 1H), 6.69 (d, J = 1.9 Hz, 1H), 3.99 (q, J = 7.0 Hz, 2H), 3.88 (s, 2H), 3.74 (s, 2H), 1.41 (dd, J = 8.9, 5.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.97, 146.49, 137.76, 128.60, 128.32, 127.57, 123.79, 122.92, 115.12, 110.16, 64.39, 52.40, 50.82, 14.68. Vmax (FTIR) 3300, 2921, 1454, 1233, 1068, 884, 848, 820, 751, 698, 569 cm-1HRMS (ESI) [M+H+]:m/z 335.0613; Calculated mass for C16H18BrNO2 is 334.050.
2.1.9. 3-Ethoxy-2-hydroxy-5-iodo-N-(benzyl)benzylamine (3b)
As a brown solid, 0.1111 g, 85%, mp 71-72 °C. 1H NMR (400 MHz, CDCl3) δ 7.36 – 7.26 (m, 5H), 7.04 (d, J = 1.8 Hz, 1H), 6.92 (d, J = 1.8 Hz, 1H), 4.03 (q, J = 7.0 Hz, 2H), 3.92 (s, 2H), 3.79 (s, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 148.17, 147.43, 137.76, 129.13, 128.64, 128.35, 127.61, 124.52, 120.87, 79.62, 64.43, 52.44, 50.63, 14.73. Vmax (FTIR) 3301, 2926, 1454, 1233, 1068, 821, 754, 700,566, 484 cm-1. HRMS (ESI) [M+H+]:m/z 384.0457; Calculated mass for C16H18INO2 is 383.040.
2.1.10. 3-Ethoxy-2-hydroxy-N-(2-picolyl)benzylamine (1c)
As a light brown solid, 0.1302 g, 84%, mp 67-70 °C. 1H NMR (400 MHz, CDCl3) δ 8.50 (d, J = 4.7 Hz, 1H), 7.57 (td, J = 7.7, 1.7 Hz, 1H), 7.16 (d, J = 7.8 Hz, 1H), 7.11 (dt, J = 11.9, 6.0 Hz, 1H), 6.78 – 6.72 (m, 1H), 6.66 (t, J = 7.8 Hz, 1H), 6.55 (d, J = 7.5 Hz, 1H), 4.02 (q, J = 7.0 Hz, 2H), 3.93 (s, 2H), 3.85 (s, 2H), 1.40 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.66, 149.03, 147.10, 146.99, 136.43, 122.78, 122.43, 122.09, 120.52, 118.40, 111.95, 63.90, 52.88, 51.14, 14.69. Vmax (FTIR) 3055, 2975, 2923, 1432, 1232, 1068, 884, 826, 762, 725, 630, 563 cm-1. HRMS (ESI) [M+H+]:m/z 259.1435; Calculated mass for C15H18N2O2 is 258.140.
2.1.11. 3-Ethoxy-2-hydroxy-5-iodo-N-(2-picolyl)benzylamine (3c)
As a brown-yellow solid, 0.1099 g, 83%, mp 67-69 °C. 1H NMR (400 MHz, CDCl3) δ 8.48 (d, J = 4.4 Hz, 1H), 7.60 – 7.52 (m, 1H), 7.18 – 7.07 (m, 3H), 6.97 (d, J = 1.7 Hz, 1H), 6.85 (s, 1H), 3.96 (q, J = 7.0 Hz, 2H), 3.87 (s, 2H), 3.83 (s, 2H), 1.37 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 149.01, 147.94, 147.25, 136.47, 136.41, 129.11, 122.46, 122.19, 122.12, 120.63, 79.39, 64.21, 52.60, 14.55. Vmax (FTIR) 3186, 2976, 1465, 1233, 1070, 886, 818, 759, 651, 568, 406cm-1. HRMS (ESI) [M+H+]:m/z 385.0414; Calculated mass for C15H17IN2O2 is 384.030.
2.1.12. 3-Ethoxy-2-hydroxy-N-(2-fluorophenyl)benzylamine (1d)
As a brown solid crystals, 0.1384 g, 88%, mp 89-91 °C.. 1H NMR (400 MHz, CDCl3) δ 7.01 – 6.93 (m, 2H), 6.92 – 6.86 (m, 1H), 6.83 – 6.77 (m, 3H), 6.67 – 6.59 (m, 1H), 4.41 (s, 2H), 4.11 (q, J = 7.0 Hz, 1H), 1.45 (t, J = 7.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 152.94, 150.57, 145.73, 143.81, 136.54, 136.42, 124.51, 124.47, 124.23, 120.89, 119.52, 116.98, 116.91, 114.40, 114.22, 112.84, 112.80, 64.50, 43.04, 14.88. Vmax (FTIR) 3429, 2923, 1463, 1268, 1048, 855, 747, 730, 519, 481, 455 cm-1HRMS (ESI) [M+H+]:m/z 262.1239; Calculated mass for C15H16FNO2 is 261.120.
2.1.13. 5-Bromo-3-ethoxy-2-hydroxy-N-(2-fluorophenyl)ben-zylamine (2c)
As a cream white solid, 0.1198 g, 86%, mp 111-112 °C. 1H NMR (400 MHz, CDCl3) δ 7.02 (d, J = 2.1 Hz, 1H), 7.00 – 6.93 (m, 2H), 6.89 (d, J = 2.2 Hz, 1H), 6.73 (dd, J = 12.5, 4.4 Hz, 1H), 6.69 – 6.61 (m, 1H), 4.37 (s, 2H), 4.08 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.02, 150.65, 146.40, 142.92, 125.82, 124.60, 124.57, 123.34, 117.60, 117.54, 114.57, 114.39, 113.99, 113.02, 111.41, 64.92, 42.86, 14.75. Vmax (FTIR) 3518, 2952, 1483, 1275, 1052, 745, 623, 512, 485, 456 cm-1. HRMS (ESI) [M+H+]:m/z 340.0344; Calculated mass for C15H15BrFNO2 is 339.03.
2.1.14. 3-Ethoxy-2-hydroxy-5-iodo-N-(2-fluorophenyl)benzy lamine (3d)
As a orange solid crystals, 0.1324 g, 86%, mp 109-111 °C. 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 1.9 Hz, 1H), 7.04 (d, J = 1.9 Hz, 1H), 7.00 – 6.93 (m, 2H), 6.77 – 6.71 (m, 1H), 6.69 – 6.62 (m, 1H), 4.34 (s, 2H), 4.07 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.04, 150.67, 146.55, 143.83, 135.87, 135.75, 129.61, 126.33, 124.60, 124.57, 119.67, 117.69, 117.62, 114.58, 114.39, 113.12, 80.86, 64.91, 42.78, 14.77. Vmax (FTIR) 3452, 1618, 1458, 1272, 1221, 1085, 824, 745, 511 cm-1. HRMS (ESI) [M+H+]:m/z 388.0216; Calculated mass for C15H15FINO2 is 387.010.
2.1.15. 3-Ethoxy-2-hydroxy-N-(2-iodophenyl)benzylamine(1e)
As a brown oil, 0.1989 g, 85%. 1H NMR (400 MHz, CDCl3) δ 7.58 (dd, J = 7.8, 1.5 Hz, 1H), 7.08 (ddt, J = 9.5, 3.6, 1.8 Hz, 1H), 6.82 – 6.76 (m, 1H), 6.73 – 6.70 (m, 2H), 6.56 (dd, J = 8.2, 1.4 Hz, 1H), 6.36 (td, J = 7.6, 1.4 Hz, 1H), 4.36 (s, 2H), 4.03 (q, J = 7.0 Hz, 2H), 1.38 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.12, 145.67, 143.67, 138.92, 129.35, 123.98, 120.60, 119.55, 118.83, 111.24, 110.53, 85.59, 64.50, 43.33, 14.91. Vmax (FTIR) 3400, 2928, 1470, 1262, 1054, 857, 736, 644, 416 cm-1. HRMS (ESI) [M+H+]:m/z 370.1025; Calculated mass for C15H16INO2 is 369,200.
2.1.16. 5-Bromo-3-ethoxy-2-hydroxy-N-(2-iodophenyl) benzy lamine (2d)
As an orange-yellow solid, 0.1639 g, 90%, mp 72-75 °C. 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 7.8, 1.2 Hz, 1H), 7.19 – 7.14 (m, 1H), 7.00 (d, J = 2.0 Hz, 1H), 6.89 (d, J = 2.0 Hz, 1H), 6.58 (d, J = 8.2 Hz, 1H), 6.49 – 6.43 (m, 1H), 4.39 (s, 2H), 4.08 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 146.74, 146.33, 142.77, 139.00, 129.42, 125.68, 123.09, 119.25, 113.88, 111.45, 111.29, 85.71, 64.90, 43.17, 14.76. Vmax (FTIR) 3421, 2922, 1486, 1230, 1090, 819, 744, 561, 419 cm-1. HRMS (ESI) [M+H+]:m/z 447.9410; Calculated mass for C15H15BrINO2 is 446.93.
2.1.17. 3-Ethoxy-2-hydroxy-5-iodo-N-(2-iodophenyl)benzyla-mine (3e)
As a brown solid, 0.1501 g, 89%, mp 78-80 °C. 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 7.8, 1.4 Hz, 1H), 7.18 (d, J = 1.6 Hz, 1H), 7.17 – 7.14 (m, 1H), 7.04 (d, J = 1.8 Hz, 1H), 6.59 (dd, J = 8.2, 1.2 Hz, 1H), 6.46 (td, J = 7.7, 1.4 Hz, 1H), 4.36 (s, 2H), 4.07 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 146.82, 146.49, 143.69, 138.99, 129.42, 129.35, 126.28, 119.54, 119.20, 111.25, 85.72, 80.92, 64.89, 43.06, 14.78. Vmax (FTIR) 3403, 2923, 1460, 1245, 1055, 1003, 895, 839, 735, 640, 575, 540,427cm-1. HRMS (ESI) [M+H+]:m/z 495.9275; Calculated mass for C15H15I2NO2 is 494.920.
2.1.18. 3-Ethoxy-2-hydroxy-N-(2-methoxyphenyl)benzyla-mine (1f)
As a brown solid, 0.1462 g, 89%, mp 102-104 °C. 1H NMR (400 MHz, CDCl3) δ 6.90 – 6.84 (m, 3H), 6.80 – 6.77 (m, 2H), 6.64 (m, 2H), 4.32 (s, 2H), 4.13 (q, J = 7.0 Hz, 2H), 1.47 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.42, 155.08, 145.89, 144.06, 124.20, 121.05, 119.54, 115.69, 115.47, 114.74, 114.67, 110.83, 64.49, 44.81, 14.89. Vmax (FTIR) 3371, 2987, 2923, 1467, 1253, 1059, 1005, 817, 763, 725, 503, 435 cm-1. HRMS (ESI) [M+H+]:m/z 274.2379; Calculated mass for C16H19NO3 is 273.140.
2.1.19. 5-Bromo-3-ethoxy-2-hydroxy-N-(2-methoxyphenyl) benzylamine (2e)
As a brown-yellow solid, 0.1302 g, 91%, mp 105-106 °C. 1H NMR (400 MHz, CDCl3) δ 6.99 (d, J = 2.2 Hz, 1H), 6.91 – 6.83 (m, 3H), 6.64 – 6.56 (m, 2H), 4.28 (s, 2H), 4.06 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.47, 155.12, 146.56, 143.82, 143.80, 143.13, 125.96, 123.31, 115.76, 115.53, 114.64, 114.57, 114.01, 111.33, 64.85, 44.35, 14.71. Vmax (FTIR) 3292, 2958, 2921, 1483, 1464, 1203, 1105, 1064, 824, 757, 727, 573, 509, 409 cm-1. HRMS (ESI) [M+H+]:m/z 353.1584; Calculated mass for C16H18BrNO3 is 352.050.
2.1.20. 3-Ethoxy-2-hydroxy-5-iodo-N-(2-methoxyphenyl)ben-zylamine (3f)
As a yellow solid, 0.1157 g, 85%, mp 111-113 °C.. 1H NMR (400 MHz, CDCl3) δ 7.20 (d, J = 1.9 Hz, 1H), 7.04 (d, J = 1.9 Hz, 1H), 6.88 – 6.81 (m, 1H), 6.81 – 6.76 (m, 2H), 6.76 – 6.69 (m, 1H), 6.66 (dt, J = 7.2, 3.6 Hz, 1H), 4.33 (s, 2H), 4.06 (q, J = 7.0 Hz, 2H), 3.84 (s, 3H), 1.46 – 1.41 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.34, 146.76, 144.22, 137.28, 129.46, 126.82, 121.20, 119.68, 117.83, 111.29, 109.46, 80.77, 64.81, 55.40, 43.52, 14.77. Vmax (FTIR) 3420, 1443, 1265, 1082, 1048, 866, 848, 780, 740, 571, 457 cm-1. HRMS (ESI) [M+H+]:m/z 400.0447; Calculated mass for C16H18INO3 is 399.030.
2.1.21. 3-Ethoxy-2-hydroxy-N-(4-fluorophenyl)benzylamine (1g)
As a cream white solid, 0.1325 g, 84%, mp 68-70 °C. 1H NMR (400 MHz, CDCl3) δ 7.28 (s, 1H), 6.95 – 6.87 (m, 3H), 6.84 – 6.80 (m, 2H), 6.70 – 6.63 (m, 2H), 4.36 (s, 2H), 4.14 (q, J = 7.0 Hz, 2H), 1.48 (t, J = 7.0, Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 156.23 (d, J = 235.4 Hz), 145.97, 144.34, 144.32, 144.12, 124.40, 121.08, 119.60, 115.74, 115.52, 114.66, 114.59, 110.87, 64.55, 44.79, 14.95. Vmax (FTIR) 3436, 2924, 1446, 1252, 1057, 815, 762, 725, 507, 447 cm-1. HRMS (ESI) [M+H+]:m/z 262.1239; Calculated mass C15H16FNO2 for is 261.120.
2.1.22. 5-Bromo-3-ethoxy-2-hydroxy-N-(4-fluorophenyl)ben-zylamine (2f)
As a yellow solid, 0.1129 g, 81%, mp 105-107 °C. 1H NMR (400 MHz, CDCl3) δ 7.00 (d, J = 2.2 Hz, 1H), 6.91 – 6.84 (m, 3H), 6.65 – 6.58 (m, 2H), 4.29 (s, 2H), 4.07 (q, J = 7.0 Hz, 2H), 1.47 – 1.42 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.57, 155.22, 146.57, 143.16, 125.87, 123.38, 115.80, 115.57, 114.78, 114.71, 114.07, 111.36, 64.88, 44.44, 14.74. Vmax (FTIR) 3292, 2920, 1241, 1066, 829, 762, 728, 580, 509 cm-1. HRMS (ESI) [M+H+]:m/z 340.0337; Calculated mass for C15H15BrFNO2 is 339.03.
2.1.23. 3-Ethoxy-2-hydroxy-5-iodo-N-(4-fluorophenyl)ben-zylamine (3g)
As a yellow solid, 0.1100 g, 83%, mp 79-81 °C. 1H NMR (400 MHz, CDCl3) δ 7.18 (d, J = 1.9 Hz, 1H), 7.03 (d, J = 1.9 Hz, 1H), 6.89 (d, J = 8.8 Hz, 2H), 6.67 – 6.58 (m, 2H), 4.26 (s, 2H), 4.06 (q, J = 7.0 Hz, 2H), 1.43 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.66, 155.31, 146.72, 144.08, 143.49, 129.65, 126.31, 119.75, 115.80, 115.58, 114.97, 114.90, 80.83, 64.86, 44.40, 14.76. Vmax (FTIR) 3377, 2922, 1466, 1200, 1094, 817, 793, 757, 729, 575, 499, 444cm-1. HRMS (ESI) [M+H+]:m/z 388.0201; Calculated mass for C15H15IFNO2 is 387.010.
2.1.24. 3-Ethoxy-2-hydroxy-N-(4-iodophenyl)benzylamine (1h)
As a limestone brown solid, 0.2001 g, 90%, mp 93-95 °C. 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 8.8 Hz, 2H), 6.88 – 6.81 (m, 2H), 6.80 – 6.72 (m, 2H), 6.52 (d, J = 8.8 Hz, 2H), 4.33 (s, 2H), 4.09 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 145.75, 143.83, 137.76, 121.17, 119.62, 116.46, 110.85, 77.20, 64.53, 43.96, 14.89. Vmax (FTIR) 3410, 2979, 1466, 1273, 1054, 847, 808, 762, 730, 558, 494 cm-1. HRMS (ESI) [M+H+]:m/z 370.0305; Calculated mass for C15H16INO2 is 370,200.
2.1.25. 5-Bromo-3-ethoxy-2-hydroxy-N-(4-iodophenyl) ben-zylamine (2g)
As a grey-green solid, 0.1721 g, 94%, mp 112-114 °C. 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 8.0, 2H), 6.98 (d, J = 2.1 Hz, 1H), 6.87 (d, J = 2.2 Hz, 1H), 6.47 – 6.40 (m, 2H), 4.28 (s, 2H), 4.07 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.19, 146.36, 142.77, 137.73, 125.81, 123.25, 115.54, 113.90, 111.40, 78.80, 64.90, 42.89, 14.74. Vmax (FTIR) 3488, 1488, 1298, 1049, 813, 785, 577, 511, 511, 483 cm-1. HRMS (ESI) [M+H+]:m/z 447.9413; Calculated mass for C15H15BrINO2 is 446.93.
2.1.26. 3-Ethoxy-2-hydroxy-5-iodo-N-(4-iodophenyl)benzyla-mine (3h)
As a white solid, 0.1330 g, 79%, mp 143-145 °C. 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 1.8 Hz, 1H), 7.02 (d, J = 1.9 Hz, 1H), 6.45 (d, J = 8.0 Hz, 2H), 4.26 (s, 2H), 4.06 (q, J = 7.0 Hz, 2H), 1.43 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 146.81, 146.54, 143.75, 137.78, 129.61, 126.11, 119.65, 115.90, 80.88, 79.35, 64.91, 43.03, 14.76. Vmax (FTIR) 3487, 1471, 1262, 1047, 812, 780, 563, 486 cm-1. HRMS (ESI) [M+H+]:m/z 495.9283; Calculated mass for C15H15I2NO2 is 494.920.
2.1.27. 3-Ethoxy-2-hydroxy-N-(4-methoxyphenyl)benzyla-mine (1i)
As a dark brown solid, 0.1402 g, 85%, mp 82-85 °C. 1H NMR (400 MHz, CDCl3) δ 6.86 – 6.84 (m, 1H), 6.80 – 6.75 (m, 4H), 6.72 – 6.67 (m, 2H), 4.33 (s, 2H), 4.09 (q, J = 7.0 Hz, 2H), 3.74 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 152.76, 146.09, 144.47, 141.79, 120.93, 119.39, 115.45, 114.65, 110.97, 64.38, 55.63, 45.81, 14.85. Vmax (FTIR) 3383, 2923, 1464, 1221, 1124, 1032, 816, 765, 720, 637, 518 cm-1. HRMS (ESI) [M+H+]:m/z 274.1428; Calculated mass for C16H19NO3 is 273.140.
2.1.28. 5-Bromo-3-ethoxy-2-hydroxy-N-(4-methoxyphenyl) benzylamine (2h)
As a brown-yellow solid, 0.1290 g, 90%, mp 82-84 °C. 1H NMR (400 MHz, CDCl3) δ 6.98 (d, J = 2.2 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 6.79 – 6.74 (m, 2H), 6.70 – 6.64 (m, 2H), 4.28 (s, 2H), 4.05 (q, J = 7.0 Hz, 2H), 3.73 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.08, 146.83, 143.68, 141.21, 125.99, 123.29, 115.66, 114.73, 114.21, 111.18, 64.77, 55.65, 45.68, 14.71. Vmax (FTIR) 3410, 2921, 1488, 1231, 1050, 1033, 820, 705, 578, 508 cm-1. HRMS (ESI) [M+H+]:m/z 352.0540; Calculated mass for C16H18BrNO3 is 351.050.
2.1.29. 3-Ethoxy-2-hydroxy-5-iodo-N-(4-methoxyphenyl)ben-zylamine (3i)
As a brown-yellow solid, 0.1199 g, 88%, mp 78-81 °C. 1H NMR (400 MHz, CDCl3) δ 7.16 (d, J = 1.9 Hz, 1H), 7.03 (d, J = 1.9 Hz, 1H), 6.77 (d, J = 8.0 Hz, 2H), 6.69 (d, J = 8.0 Hz, 2H), 4.27 (s, 2H), 4.05 (q, J = 7.0 Hz, 2H), 3.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 153.30, 147.01, 144.63, 140.88, 129.60, 126.38, 119.97, 115.94, 114.75, 80.72, 64.79, 55.67, 45.71, 14.76. Vmax (FTIR) 3507, 2965, 1466, 1218, 1053, 1031, 818, 522, 423 cm-1. HRMS (ESI) [M+H+]:m/z 400.0397; Calculated mass for C16H18INO3 is 399.030.
2.1.30. 3-Ethoxy-2-hydroxy-N-(2-bromo-4-methylphenyl)ben-zylamine (1j)
As a brown solid, 0.2032 g, 95%, mp 67-69 °C. 1H NMR (400 MHz, CDCl3) δ 7.30 (d, J = 1.4 Hz, 1H), 7.00 – 6.95 (m, 1H), 6.90 (dt, J = 6.5, 3.3 Hz, 1H), 6.84 – 6.80 (m, 2H), 6.66 (d, J = 8.2 Hz, 1H), 4.45 (s, 2H), 4.14 (q, J = 7.0 Hz, 2H), 2.24 (s, 3H), 1.49 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 145.72, 143.78, 142.46, 132.67, 128.94, 127.78, 124.15, 120.67, 119.52, 112.21, 110.58, 110.03, 64.49, 43.50, 20.01, 14.90. Vmax (FTIR) 3534, 2921, 2852, 1463, 1273, 1058, 861, 800, 722, 549, 424 cm-1. HRMS (ESI) [M+H+]:m/z 336.0570; Calculated mass for C16H18BrNO2 is 335.050.
2.1.31. 3-Ethoxy-2-hydroxy-5-iodo-N-(2-bromo-4-methylp-henyl) benzylamine (3j)
As a peach solid, 2.21 g, 98%, mp 99-101 °C. 1H NMR (400 MHz, CDCl3) δ 7.26 (d, J = 1.4 Hz, 1H), 7.18 (d, J = 1.9 Hz, 1H), 7.03 (d, J = 1.9 Hz, 1H), 6.94 (dd, J = 8.2, 1.4 Hz, 1H), 6.59 (d, J = 8.2 Hz, 1H), 4.34 (s, 2H), 4.06 (q, J = 7.0 Hz, 2H), 2.21 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H). Vmax (FTIR) 3404, 1471, 1273, 1052, 860, 805, 777, 719, 673, 559, 526 cm-1. HRMS (ESI) [M+H+]:m/z 447.2945; Calculated mass for C15H15BrINO2 is 446.930.
2.1.32. Methyl3-bromo-4-((3-ethoxy-2-hydroxybenzyl)amino) benzoate (1k)
As a yellow solid, 0.2020 g, 89%, mp 89-91 °C. 1H NMR (400 MHz, MeOD) δ 7.99 (d, J = 1.9 Hz, 1H), 7.71 – 7.67 (m, 1H), 6.91 (dd, J = 7.4, 1.7 Hz, 1H), 6.84 – 6.73 (m, 4H), 4.94 (s, 6H), 4.68 (s, 2H), 4.05 (q, J = 7.0 Hz, 3H), 3.82 (s, 3H), 1.40 (t, J = 7.0 Hz, 4H).13C NMR (101 MHz, MeOD) δ 168.11, 151.69, 147.80, 145.50, 135.63, 131.42, 128.84, 121.59, 120.38, 120.14, 115.30, 112.89, 108.04, 65.78, 60.87, 52.57, 15.42. Vmax (FTIR) 3462, 2982, 1689, 1471, 1247, 1114, 1070, 829, 763, 737, 632, 436 cm-1. HRMS (ESI) [M+H+]:m/z 380.0502; Calculated mass for C17H18BrNO4 is 379.040.
2.1.33. 3-Ethoxy-2-hydroxy-N-(5-Chloro-2-iodophenyl)ben-zylamine (1l)
As a peach solid, 0.2034 g, 83%, mp 68-70 °C. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.3 Hz, 1H), 6.85 (dt, J = 9.5, 3.9 Hz, 1H), 6.82 – 6.79 (m, 2H), 6.63 (d, J = 2.3 Hz, 1H), 6.43 (dd, J = 8.3, 2.3 Hz, 1H), 4.39 (s, 2H), 4.12 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 148.02, 145.69, 143.69, 139.38, 135.49, 123.26, 120.69, 119.69, 118.63, 111.02, 110.73, 82.37, 64.56, 43.22, 14.91. Vmax (FTIR) 3478, 2954, 1482, 1220, 1080, 831, 765, 725, 644, 505, 435 cm-1. HRMS (ESI) [M+H+]:m/z 404.9954; Calculated mass for C15H15ClINO2 is 402.980.
2.1.34. 5-Bromo-3-ethoxy-2-hydroxy-N-(5-chloro-2-iodophe-nyl) benzylamine (2i)
As a brown solid, 0.1679 g, 85%, mp 96-99 °C. 1H NMR (400 MHz, CDCl3) δ 7.57 – 7.51 (m, 1H), 6.97 (d, J = 2.1 Hz, 1H), 6.90 (d, J = 2.1 Hz, 1H), 6.54 (d, J = 2.3 Hz, 1H), 6.44 (dd, J = 8.3, 2.3 Hz, 1H), 4.35 (s, 2H), 4.13 – 4.05 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.81, 146.32, 142.71, 139.44, 135.52, 125.08, 123.02, 118.85, 114.04, 111.54, 110.82, 82.36, 64.97, 42.72, 14.75. Vmax (FTIR) 3377, 2921, 1455, 1280, 1080, 1007, 822, 786, 721, 576, 429 cm-1. HRMS (ESI) [M+H+]:m/z 482.8921; Calculated mass for C15H14BrClINO2 is 480.890.
2.1.35. 3-Ethoxy-2-hydroxy-5-iodo-N-(5-chloro-2-iodophe-nyl)) benzylamine (3k)
As a yellow solid, 0.1502 g, 83%, mp 70-73 °C. 1H NMR (400 MHz, CDCl3) δ 7.55 – 7.48 (m, 2H), 7.16 (d, J = 1.8 Hz, 1H), 7.05 (d, J = 1.8 Hz, 1H), 6.72 (d, J = 2.3 Hz, 1H), 6.54 (d, J = 2.3 Hz, 1H), 6.45 (td, J = 8.1, 2.3 Hz, 2H), 4.32 (s, 2H), 4.08 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 147.86, 146.48, 143.63, 139.60, 139.44, 135.52, 129.33, 125.67, 119.93, 119.69, 118.83, 114.20, 110.82, 82.37, 81.00, 80.98, 64.96, 42.61, 14.77. Vmax (FTIR) 3458, 2921, 1271, 1070, 838, 774, 734, 690, 646, 569, 492, 437 cm-1. HRMS (ESI) [M+H+]:m/z 530.4001; Calculated mass for C15H14ClI2NO2 is 528.880.
2.1.36. 3-Ethoxy-2-hydroxy-N-(5-fluoro-2-iodophenyl) benzy-lamine (1m)
As a light brown solid, 0.1981 g, 85%, mp 74-76 °C. 1H NMR (400 MHz, CDCl3) δ 7.54 (dt, J = 8.7, 6.2 Hz, 2H), 6.88 – 6.82 (m, 1H), 6.82 – 6.76 (m, 2H), 6.45 (dd, J = 10.5, 2.8 Hz, 1H), 6.36 (dd, J = 11.7, 2.8 Hz, 1H), 4.40 (s, 2H), 4.11 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 165.55, 165.14, 163.13, 162.71, 148.68, 148.57, 148.09, 147.98, 145.65, 143.60, 139.63, 139.53, 139.26, 139.16, 123.38, 120.55, 119.63, 110.61, 107.24, 107.01, 105.48, 105.26, 101.59, 101.34, 98.60, 98.33, 77.95, 77.92, 64.51, 43.02, 14.87. Vmax (FTIR) 3405, 2921, 1455, 1260, 1055, 856, 828, 763, 734, 588, 516, 438 cm-1. HRMS (ESI) [M+H+]:m/z 388.0225; Calculated mass for C15H15FINO2 is 387.010.
2.1.37. 5-Bromo-3-ethoxy-2-hydroxy-N-(5-fluoro-2-iodophe-nyl)benzylamine (2j)
As a yellow solid, 0.1601 g, 84%, mp 83-85 °C1H NMR (400 MHz, CDCl3) δ 7.56 (dd, J = 8.6, 6.3 Hz, 1H), 6.97 (d, J = 2.1 Hz, 1H), 6.89 (d, J = 2.2 Hz, 1H), 6.29 (dd, J = 11.6, 2.8 Hz, 1H), 6.20 (ddd, J = 11.8, 8.7, 3.9 Hz, 1H), 4.35 (s, 2H), 4.08 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 165.54, 163.12, 148.37, 148.26, 146.30, 142.65, 139.37, 139.28, 125.13, 122.93, 113.95, 111.53, 105.84, 105.62, 98.60, 98.33, 78.00, 77.97, 64.94, 42.66, 14.73. Vmax (FTIR) 3476, 2922, 1453, 1269, 1091, 816, 775, 559, 464 cm-1. HRMS (ESI) [M+H+]:m/z 467.9312; Calculated mass for C15H14BrFINO2 is 466.091.
2.1.38. 3-Ethoxy-2-hydroxy-N-(3,5-dichlorophenyl)benzyla-mine (1n)
As a white solid, 0.1632 g, 87%, mp 88-91 °C. 1H NMR (400 MHz, CDCl3) δ 6.85 – 6.81 (m, 1H), 6.80 – 6.76 (m, 2H), 6.64 (t, J = 1.7 Hz, 1H), 6.52 (d, J = 1.7 Hz, 2H), 4.30 (s, 2H), 4.11 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 149.68, 145.72, 143.69, 135.30, 123.51, 121.00, 119.69, 117.15, 111.30, 110.80, 64.57, 43.04, 14.89. Vmax (FTIR) 3445, 2980, 1468, 1271, 1058, 796, 737, 667, 630, 562, 499, 476, 435 cm-1. HRMS (ESI) [M+H+]:m/z 312.0563; Calculated mass for C15H15Cl2NO2 is 311.050.
2.1.39. 5-Bromo-3-ethoxy-2-hydroxy-N-(3,5-dichlorophenyl) benzylamine (2k)
As a cream white solid, 0.1413 g, 89%, mp 121-123 °C. 1H NMR (400 MHz, CDCl3) δ 6.98 (d, J = 2.1 Hz, 1H), 6.89 (d, J = 2.2 Hz, 1H), 6.69 (t, J = 1.7 Hz, 1H), 6.53 (d, J = 1.7 Hz, 2H), 4.27 (s, 2H), 4.09 (q, J = 7.0 Hz, 2H), 1.45 (dd, J = 8.8, 5.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 146.37, 142.77, 135.43, 124.93, 123.34, 117.96, 114.16, 111.69, 111.52, 65.00, 42.76, 14.74. Vmax (FTIR) 3426, 2923, 1456, 1274, 1068, 892, 821, 796, 667, 582, 496, 476 cm-1. HRMS (ESI) [M+H+]:m/z 389.9667; Calculated mass for C15H14BrCl2NO2 is 388.960.
2.1.40. 3-Ethoxy-2-hydroxy-5-iodo-N-(3,5-dichlorophenyl)) benzylamine (3l)
As a cream white solid, 0.1301 g, 86%, mp 108-110 °C. 1H NMR (400 MHz, CDCl3) δ 7.15 (d, J = 1.9 Hz, 1H), 7.04 (d, J = 1.9 Hz, 1H), 6.66 (t, J = 1.7 Hz, 1H), 6.48 (d, J = 1.7 Hz, 2H), 4.24 (s, 2H), 4.06 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 149.37, 146.46, 143.56, 135.33, 129.46, 125.80, 119.65, 117.37, 111.20, 80.95, 64.94, 42.25, 14.74. Vmax (FTIR) 3370, 2919, 1442, 1262, 1102, 1055, 804, 667, 510, 463 cm-1. HRMS (ESI) [M+H+]:m/z 400.3279; Calculated mass for C15H14Cl2INO2 is 399.030.
3. RESULTS AND DISCUSSIONS
3.1. Chemistry
To access the compounds with antitubercular properties, 3-ethoxysalcyladehyde 1 served as the starting point of the investigations. The investigation started by iodinating the starting material using N-iodosuccinimide (NIS) according to the reported synthetic method. This method was also used for the bromination of the starting material using N-bromosuccinimide (NBS) resulting in the formation of products 2 and 3, respectively (Scheme 1) [33]. Reductive amination is a very useful synthetic method that was used to access pharmaceutical drugs used in the treatment of various diseases such as cancer, diabetes, fungal infections and many more [34]. Compounds 1 – 3 were treated with various aromatic amines under reductive amination reaction conditions using sodium borohydride in methanol to yield benzylamine derivatives as shown in Scheme. (1) [34].

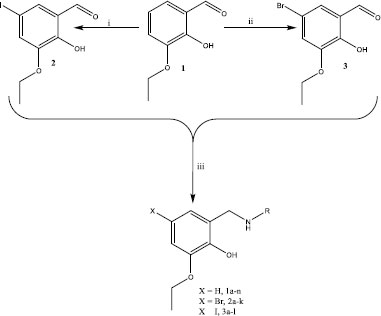
Compounds 1a-n were synthesised by treating 3-ethoxysalcyladehyde 1 with various aromatic amines and were obtained in good to excellent yields. The 1H NMR spectra of the compounds showed the disappearance of the aldehyde signal around 10 ppm and the appearance of the methylene signals ranging from 3.93 – 4.68 ppm in addition to other proton signals from the aromatic amines. To supplement the 1H NMR spectroscopy, the 13C NMR spectra were recorded. The 13C NMR spectra of 1a-n showed the disappearance of the aldehyde signal around 196 ppm and the appearance of the methylene signals in the region of 43.04 – 52.57 ppm. The same aromatic amines used in the synthesis of compounds 1a – n were also used for the construction of compounds 2a – k and 3a-l and thus displayed similar NMR spectroscopic characteristics. For example, in the case of compounds 2a – k 1H NMR spectra, methylene signals appeared in the region of 3.88 – 4.39 ppm while compounds 3a – l showed methylene signals ranging from 3.83 ppm to 4.36 ppm. The 13NMR spectra signals of the methylene group appeared between 42.66 and 52.40 ppm for compounds 2a – k and between 42.25 and 52.60 ppm for compounds 3a – l. The successful synthesis of all the compounds was confirmed by both 1H and 13C NMR, IR and mass spectroscopy.
3.2. Biological Evaluation
All the synthesised compounds were evaluated for their biological activity against Myocobacterium tuberculosis (Mtb) H37RV strain. The biological assays were performed following a broth dilution method in 7H9_ADC_GLU_TW (7 days) and rifampicin was used as a positive control [35]. The results for the in vitro biological assays are displayed in Table 1. The biological assay results in Table 1 indicate that all compounds possessed activity against Myocobacterium tuberculosis with MIC90 values ranging from 20.04 (most active) – > 125 µM (least active). Only one compound, 2f was considered ineffective with an MIC90 value >125 µM. Other compounds in this series exhibited better activity with MIC90 values of < 30 µM. The most active compounds in this series, 2c and 2e exhibited MIC90 values of 20.04 µM representing the most active compounds for 2-fluorophenyl and 2-methoxyphenyl substituents, respectively.
When phenyl, benzyl, 2-picolyl and 2-iodophenyl were used as substituents, the most active compounds were 2a (22.26 µM), 2b (20.59 µM), 1c (20.44 µM) and 3e (20.29 µM), respectively. The use of ortho-substituted phenyl reagents has produced some of the most active compounds in the series while the use of para-substituted phenyl reagents produced compounds with reduced activity against Mycobacterium tuberculosis. For example, 2f with 4-fluorophenyl substituent was inactive, 2g with 4-iodophenyl substituent exhibited an activity of 20.39 µM while 4-methoxypheyl (3i) had an activity of 21.89 µM. The reduced activity against Mtb was further observed when some disubstituted phenyl reagents were used. Compound 3j displayed an activity of 21.54 µM, compound 1k showed an activity of 21.21 µM and compound 3k had the best activity at 21.84 µM. These MIC90 values are slightly lower than those obtained for ortho and para substituted phenyl derivatives. Notably, 5-fluoro-2-iodophenyl and 3,5-dichlorophenyl substituents displayed MIC90 values comparable to those of ortho substituted phenyl substituents. The most active compounds were 3l (20.59 µM), followed by compound 1m (20.64 µM) and lastly compound 1n (20.67 µM).
In addition to the biological assays against Myoco-bacterium tuberculosis, cytotoxicity assays were also performed for all the compounds against Chinese Hamster Ovarian (CHO) cells to determine the concentration at which these compounds are likely to be toxic. Quantitative assessment of toxic activity in vitro was determined via the MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide] assay with emetine used as a standard [36]. The test compounds were largely non-toxic to CHO cells up to the highest concentration evaluated of 50 µM. Only two compounds, 3c (12.45 µM) and 3h (8.80 µM) showed moderate activity against the cells, with three other compounds (3d, 47.28 µM; 3i, 44.98 µM and 3j, 48.00 µM) showing low levels of toxicity. Interestingly, all the compounds showing weak to moderate activity against CHO cells contain iodo substituent at position 5 of the benzylamine. However, not all compounds containing 5-iodo substituent displayed toxicity towards CHO cells, indicating that the whole structure of the compound is likely responsible for the activity observed.
3.3. Structure-activity Relationship and ADMET Predictions
The effect of substituents on the activity and cytotoxicity of the synthesised compounds was examined. Compounds containing 5-bromo substituent displayed the best activity against Myocobacterium tuberculosis. Notably, the activity of these compounds also depended on the type of substituents in the phenyl group. For example, ortho-substituted phenyl-containing compounds (2c, 2e) were the most active against Mtb with the exception of 2d which showed reduced activity. Although compounds containing 5-bromo substituent continued their activity against Mtb (2a, 2b, 2g), the only inactive compound in this study 2f with 4-fluorophenyl belonged to the 5-bromo substituent series. Compounds containing 5-iodo substituent displayed slightly reduced activity that largely depended on the substituents in the phenyl group. For example, compounds with 2-iodophenyl (3e), 4-fluorophenyl (3g), 4-methoxyphenyl (3i), 5-chloro-2-iodophenyl (3k) and 3,5-dichlorophenyl (3l) were the more active against Mtb in comparison to compounds containing no substituent (1e-n) and other 5-bromo substituted benzylamine derivatives (2e-k). Unfortunately, the series of compounds containing 5-iodo substituent produced the only compounds in this study that showed activity against CHO cells (3c, 3d, 3h, 3i and 3j).
Physiochemical properties (Adsorption, Distribution, Metabolism, Excretion and Toxicity (ADMET)) of the synthesised compounds were analysed using the online prediction software ADMETlab 2.0 [37, 38]. These physiochemical predictions will help in understanding the behaviour of the synthesised compounds after consumption and the results are displayed in Table 2. Most of the compounds in this synthetic series (Table 2) showed poor LogS values with the exception of compounds 1a-d, 1f, 1g, 1i, 2a, 2b, 2f, 2g, 3b, and 3c while only three compounds (1b, 1c, and 3c) possessed favourable LogP values. Additionally, only five compounds (1b, 1c, 3c, 3h, and 3l) possessed LogD values that were within the predicted values. Moreover, most of the compounds were predicted to be nontoxic with the exception of compounds 1c and 3c both containing a picolyl substituent. Interestingly, all derivatives of 2 and 3 possessed poor clearance values while some of the derivatives of 1 including 1a-d, 1f, 1g, and i possessed moderate clearance values. Furthermore, all compounds displayed poor half-life values and poor to fair protein plasma binding (PPB) values. For example, the compounds with the best half-life values (T1/2 > 0.7) were 1a-f, 1i, 1k, 2a, 2b, 2e, and 3c while compounds 1b-c and 3b-c were the only compounds to possess favourable protein plasma binding properties. A number of hydrogen acceptors (nHA) and number of hydrogen donors (nHD) of all compounds were within the predicted values.
 |
|||||||
| R = | X = | MIC90 | IC50 | R = | X = | MIC90 | IC50 |
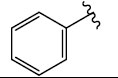 |
H (1a) | 22.66 | >50 | 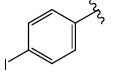 |
H (1h) | 21.00 | >50 |
| Br (2a) | 22.26 | >50 | Br (2g) | 20.39 | >50 | ||
| I (3a) | 25.45 | >50 | I (3h) | 22.50 | 8.8 | ||
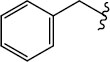 |
H (1b) | 20.78 | >50 | 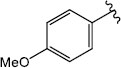 |
H (1i) | 25.79 | >50 |
| Br (2b) | 20.59 | >50 | Br (2h) | 22.66 | >50 | ||
| I (3b) | 29.62 | >50 | I (3i) | 21.89 | 44.9 | ||
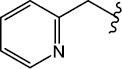 |
H (1c) | 20.44 | >50 | 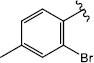 |
H (1j) | 25.79 | >50 |
| I (3c) | 22.22 | 12.5 | I (3j) | 21.54 | 48.0 | ||
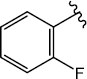 |
H (1d) | 20.35 | >50 | 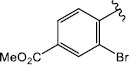 |
H (1k) | 21.21 | >50 |
| Br (2c) | 20.04 | >50 | |||||
| I (3d) | 20.68 | 47.3 | |||||
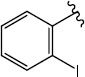 |
H (1e) | 22.95 | >50 | 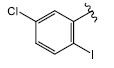 |
H (1l) | 21.94 | >50 |
| Br (2d) | 22.94 | >50 | Br (2i) | 22.66 | >50 | ||
| I (3e) | 20.29 | >50 | I (3k) | 21.84 | >50 | ||
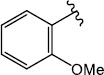 |
H (1f) | 25.79 | >50 | 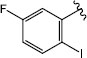 |
H (1m) | 20.64 | >50 |
| Br (2e) | 20.04 | >50 | Br (2j) | 22.05 | >50 | ||
| I (3f) | 25.02 | >50 | |||||
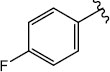 |
H (1g) | 27.73 | >50 | 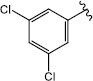 |
H (1n) | 20.67 | >50 |
| Br (2f) | >125 | >50 | Br (2k) | 25.74 | >50 | ||
| I (3g) | 22.62 | >50 | I (3l) | 20.59 | >50 | ||
| Rifampicin | - | 0.01 | - | Emetine | - | - | 0.01 |
| ID | MW | LogS | LogP | LogD | H-HT | CL | T1/2 |
PPB (%) |
nHA | nHD | Rules satisfied | ||
| GSK | Pfizer | Lipinski | |||||||||||
| 1a | 243.13 | -2.51 | 3.02 | 3.01 | -1 | 7.56 | 0.861 | 98.22 | 3 | 2 | Yes | No | Yes |
| 2a | 321.040 | -3.27 | 3.79 | 3.44 | -1 | 2.04 | 0.757 | 98.44 | 3 | 2 | Yes | No | Yes |
| 3a | 369.020 | -4.10 | 4.06 | 3.30 | -1 | 2.85 | 0.540 | 99.93 | 3 | 2 | Yes | No | Yes |
| 1b | 257.140 | -1.82 | 2.57 | 2.71 | -1 | 9.78 | 0.862 | 87.18 | 3 | 2 | Yes | Yes | Yes |
| 2b | 335.050 | -2.61 | 3.47 | 3.31 | -1 | 2.12 | 0.754 | 93.34 | 3 | 2 | Yes | No | Yes |
| 3b | 383.040 | -3.42 | 3.77 | 2.91 | -1 | 4.11 | 0.542 | 88.20 | 3 | 2 | Yes | No | Yes |
| 1c | 258.140 | -0.08 | 1.40 | 1.43 | +1 | 6.85 | 0.865 | 49.62 | 4 | 2 | Yes | Yes | Yes |
| 3c | 384.030 | -2.06 | 2.80 | 2.22 | +1 | 2.93 | 0.755 | 56.48 | 4 | 2 | Yes | No | Yes |
| 1d | 261.120 | -3.16 | 3.25 | 3.25 | -1 | 7.15 | 0.767 | 98.87 | 4 | 2 | Yes | No | Yes |
| 2c | 339.030 | -4.09 | 3.99 | 3.61 | -1 | 2.11 | 0.577 | 99.05 | 4 | 2 | Yes | No | Yes |
| 3d | 387.010 | -4.57 | 4.24 | 3.56 | -1 | 2.94 | 0.328 | 100.48 | 4 | 2 | No | No | Yes |
| 1e | 369.020 | -4.05 | 3.96 | 3.70 | -1 | 4.06 | 0.712 | 99.57 | 3 | 2 | Yes | No | Yes |
| 2d | 446.93 | -4.76 | 4.56 | 3.71 | -1 | 1.69 | 0.459 | 99.64 | 3 | 2 | No | No | Yes |
| 3e | 494.920 | -4.85 | 4.79 | 3.46 | -1 | 2.24 | 0.252 | 100.56 | 3 | 2 | No | No | Yes |
| 1f | 273.140 | -3.41 | 3.06 | 3.18 | -1 | 8.11 | 0.876 | 98.39 | 4 | 2 | Yes | No | Yes |
| 2e | 351.050 | -4.31 | 3.83 | 3.57 | -1 | 2.33 | 0.794 | 98.63 | 4 | 2 | Yes | No | Yes |
| 3f | 399.030 | -4.71 | 4.10 | 3.58 | -1 | 3.51 | 0.612 | 99.94 | 4 | 2 | No | No | Yes |
| 1g | 261.120 | -2.73 | 3.17 | 3.16 | -1 | 8.60 | 0.595 | 98.93 | 4 | 2 | Yes | No | Yes |
| 2f | 339.030 | -3.59 | 3.91 | 3.46 | -1 | 2.39 | 0.380 | 98.97 | 4 | 2 | Yes | No | Yes |
| 3g | 387.010 | -4.33 | 4.18 | 3.36 | -1 | 3.21 | 0.196 | 100.54 | 4 | 2 | No | No | Yes |
| 1h | 369.020 | -4.06 | 4.09 | 3.41 | -1 | 2.74 | 0.411 | 100.00 | 3 | 2 | No | No | Yes |
| 2g | 446.93 | -4.78 | 4.65 | 3.20 | -1 | 1.63 | 0.214 | 100.05 | 3 | 2 | No | No | Yes |
| 3h | 494.920 | -4.93 | 4.90 | 2.95 | -1 | 1.96 | 0.115 | 100.94 | 3 | 2 | No | No | Yes |
| 1i | 273.140 | -2.81 | 3.03 | 3.01 | -1 | 8.84 | 0.802 | 98.74 | 4 | 2 | Yes | No | Yes |
| 2h | 351.050 | -3.75 | 3.86 | 3.43 | -1 | 2.55 | 0.651 | 98.91 | 4 | 2 | Yes | No | Yes |
| 3i | 399.030 | 4.13 | 4.13 | 3.41 | -1 | 3.55 | 0.364 | 100.26 | 4 | 2 | No | No | Yes |
| 1j | 335.050 | -4.49 | 4.00 | 3.72 | -1 | 2.82 | 0.697 | 98.98 | 3 | 2 | No | No | Yes |
| 3j | 446.930 | -5.44 | 4.65 | 3.35 | -1 | 2.77 | 0.412 | 100.22 | 3 | 2 | No | No | Yes |
| 1k | 379.040 | -4.59 | 3.77 | 3.49 | -1 | 3.70 | 0.860 | 99.45 | 5 | 2 | Yes | No | Yes |
| 1l | 402.980 | -4.56 | 4.51 | 3.97 | -1 | 3.42 | 0.371 | 100.48 | 3 | 2 | No | No | Yes |
| 2i | 480.890 | -5.18 | 5.23 | 3.51 | -1 | 1.82 | 0.218 | 100.55 | 3 | 2 | No | No | Yes |
| 3k | 528.880 | -5.06 | 5.47 | 3.30 | -1 | 2.26 | 0.137 | 101.50 | 3 | 2 | No | No | No |
| 1m | 387.010 | -4.26 | 4.11 | 3.76 | -1 | 4.67 | 0.346 | 100.46 | 4 | 2 | No | No | Yes |
| 2j | 464.920 | -4.94 | 4.74 | 3.71 | -1 | 2.01 | 0.215 | 100.30 | 4 | 2 | No | No | Yes |
| 1n | 311.050 | -4.15 | 4.26 | 3.52 | -1 | 4.84 | 0.511 | 100.02 | 3 | 2 | No | No | Yes |
| 2k | 388.96 | -5.02 | 4.93 | 3.18 | -1 | 2.27 | 0.314 | 100.38 | 3 | 2 | No | No | Yes |
| 3l | 436.940 | -5.18 | 5.15 | 2.98 | -1 | 2.62 | 0.196 | 101.19 | 3 | 2 | No | No | Yes |
| Rif. | 882.40 | -2.35 | 3.32 | 1.89 | +1 | 1.74 | 0.50 | 77.65 | 16 | 6 | No | Yes | No |
Further analysis of Table 2 results revealed that only two compounds (1b, 1c) possessed the best predicted drug-like properties followed by 3b and 3c, which displayed poor predicted logP and clearance values respectively. In general, the use of phenyl substituents with or without halogen substituents (Br, Cl, F, I) led to poor drug-like predicted properties (e.g. 1a, 1f). Notably, the compounds with the best predicted drug-like properties possessed a methylene bridge without halogen substituents (1b and 1c), thus, indicating its role in improving drug-like properties. All compounds satisfied Lipinski’s rule with the exception of compound 3k which also failed both Pfizer’s and GSK’s rules. In addition to 3k, several compounds (e.g. 2d, 3d, 3e and others) containing more than two halogen substituents (with the exception of 3f and 3i), failed both Pfizer’s and GSK’s rules. Finally, the predicted ADMET properties of Rifampicin, the approved antitubercular drug used as a reference showed a compound with favourable logP, logD and PPB values. However, Rifampicin was predicted to have high toxicity, poor clearance and half-life values, and it was also rejected by GSK’s rule [39-41].
CONCLUSION
A total of 37 compounds were synthesised and successfully biologically evaluated against Mycobacterium tuberculosis (Mtb) H37RV strain. All compounds showed activity against Mtb at concentrations of > 20 µM < 28 µM with the exception of compound 2f which was active against Mtb at higher concentrations (MIC90 > 125 µM). The most active compounds, 2c and 2e possessed MIC90 values of 20.04 µM each. Compounds with 5-bromo substituent were the most active against Mtb. Cytotoxicity results revealed compounds largely inactive against CHO cells with the exception of 3c, 3d, 3h, 3i and 3j with 3i being the most active with an MIC50 value of 8.80 µM. Predicted ADMET properties of the synthesised compounds revealed that phenyl substituents and the presence of halogen substituents received unfavourable physiochemical properties while the introduction of methylene bridge received favourable physiochemical properties. This study has demonstrated the ability of benzylamine derivatives as possible future drug candidates for the treatment of tuberculosis.
LIST OF ABBREVIATIONS
| TB | = Tuberculosis |
| WHO | = World Health Organization |
| MD-R TB | = Multidrug-resistance tuberculosis |
| XDR-TB | = Extensively drug-resistance tuberculosis |
| CHO | = Chinese Hamster ovarian |
| NIS | = N-iodosuccinimide |
| NBS | = N-bromosuccinimide |
| Mtb | = Myocobacterium tuberculosis |
| ADMET | = Adsorption, Distribution, Metabolism, Excretion and Toxicity |
| nHA | = Number of hydrogen acceptors |
| nHD | = Number of hydrogen donors |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The authors are thankful for financial support on this project from the University of Limpopo and the National Research Foundation (South Africa) through grant number TTK180412320177.
COMPETING INTEREST
The authors declare that they have no competing financial or personal interests.
ACKNOWLEDGEMENTS
The authors wish to thank the University of Limpopo, Ms. Lerato Raphoko (University of Limpopo) for mass spectrometric data and the Drug Discovery and Development Centre (H3D) at the University of Cape Town for Mtb and cytotoxicity assay data.


