REVIEW ARTICLE
Pongamia Pinnata: An Heirloom Herbal Medicine
Ishtiaq Jeelani1, *, Tanzeela Qadir2, Alisha Sheikh3, Mrinalini Bhosale4, Praveen Kumar Sharma2, *, Andleeb Amin2, Allah Nawaz1, Aamir Sharif5, Abdul Hayee6, Muhammad Bilal7, Muhammad Rahil Aslam7, Bilal Ahmed Mir1
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e18741045240484
Publisher ID: e18741045240484
DOI: 10.2174/0118741045240484231009141434
Article History:
Received Date: 20/12/2022Revision Received Date: 29/04/2023
Acceptance Date: 14/06/2023
Electronic publication date: 23/10/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pongamia pinnata (L) is one of many plants which possess various medicinal properties, and its chemical constituents have been used for the prevention and treatment of various human diseases in many countries as traditional medicine. It contains a wide range of phytoconstituents and has a wide range of pharmacological effects. It contains glycosides, alkaloids, flavonoids, carbohydrates, and fixed oils, among other phytoconstituents. Pongamia pinnata (P. pinnata) roots are useful for cleaning teeth, treating ulcers, strengthening gum, and gonorrhea. The root paste is applied locally to treat scrofulous enlargement. Pongamia Pinnata fresh bark is fragrant and mucilaginous, but it quickly becomes bitter and caustic. Its anthelmintic nature helps in treating ophthalmic, dermal, and vaginal ulcers. P. pinnata leaves are digestive, laxative, and anthelmintic, and they can help in treating diarrhea, leprosy, dyspepsia, and cough. Flowers are useful in diabetes dypsia, as well as vata and Kapha imbalances. Its oil is used to make biodiesel as well. Therefore, this review aims to compile the latest information about P. pinnata, especially its phytochemistry, uses in folk and traditional medicines along with toxicity study, and its role in the biofuel industry to address gaps between previous and current research and to develop new research opportunities.
1. INTRODUCTION
The 'Pongam Tree' is considered one of India's richest and brightest trees. The scientific name for the tree is 'Pongamia pinnata'. The name 'Pongamia' comes from the Tamil word 'pinnata,' which means 'Pinnate leaves. 'This is referred to as 'Ponga,' 'Dalkaramacha,' 'Pongam,' and 'Punku' in Tamil. People called it 'Karanj', 'Papar', or 'Kanji' in both Hindi and Bengali. In English, it is known as the 'Karum Tree' or the 'Poonga Oil Tree.'It belongs to the family Leguminosae and 'Papilionaceae' is its subfamily. P. pinnata has a large native distribution in Australia and Asia. Additionally, the species is grown in nations like Africa, the US, and others. In India, it grows wild in coastal forests and alongside streams and rivers [1]. The 'Pongam Tree' is medium-sized and has a fast-growing nature. According to a recent study, the tree has a lot of potential for reforesting in degraded or damaged landscapes. The bark of the tree is tough and grey-brown. In this tree, new leaves appear, and numerous flowers begin to bloom at the same time. The flowers are 1.3 cm long and clump together at the ends of the long stems. These stems emerge from the leaf's top angle. There is a very short stalk on the flowers. They have a calyx that is fashioned like a cup and is loose and brown in hue. There are five white petals as well as five pink- or violet-colored petals. P. pinnata has a long history of use, which has prompted scientists to investigate its pharmacological properties and justify its use as a treatment. This plant has antioxidant, antiparasitic, antimicrobial, antidiabetic, antic cancer, anti-inflammatory, anticonvulsant, anti-hyperammonaemia, cytotoxic, anthelmintic, and insecticidal properties [2].
P. pinnata is a multi-purpose legume tree native to the Indian subcontinent and Southeast Asia. It is one of the few non-edible oil-producing trees with a significant seed yield (20,000 seeds per tree) [3]. In traditional medicine, various parts of this plant had been used to treat a variety of ailments and wounds. Prof. Limayein conducted the first investigation of this species in 1925. The root of the P. pinnata plant is used to treat urethritis and skin conditions [3].
This review seeks to provide a comprehensive understanding of the chemicals present, their phytochemistry, and their toxicity to address gaps in previous and current research and to develop new research opportunities. The origin, qualities, and biological activity of the P. pinnata plant have been detailed in this article [3].
2. TAXONOMIC CLASSIFICATION PONGAMIA PINNATA
Fabaceae, the biggest flowering plant family with 714 genera, is sometimes known as Leguminosae and is a commercially important family. The vast diversity in this family attracts many ecological and systematic investigations due to its importance as a food source and forage [1, 3] (Table 1).
| Kingdom | Plantae |
|---|---|
| Sub-kingdom | Tracheobionta |
| Super-division | Spermatophya |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Rosidae |
| Order | Fabales |
| Family | Fabaceae |
| Genus | Pongamia |
| Species | Pinnata |
| Sanskrit | Ghrtakarauja, Karanjaka, Naktahva, Naktamala |
|---|---|
| Bengali | Daharakaranja, Karanja, Natakaranja |
| Assamese | Korach |
| Kannada | Honge, Hulagilu |
| Marathi | Karanja |
| Gujrati | Kanaji, Kanajo |
| Punjabi | Kranj |
| Telugu | Ganuga, Kanugu |
| Hindi | Karuaini, Dithouri |
| Urdu | Karanj |
| Malayalam | Pungu, Ungu, Unu, Avittal |
| Oriya | Karanja |
| Tamil | Pungai, Pongana |
P. pinnata is the only species of the genus Pongamia [3]. Pinnata Pongamia is a member of the Fabaceae family. It's also known as Pongamia glabra, and it's a fast-growing evergreen tree that grows up to 40 feet tall and spreads out to form a broad canopy that provides mild shade. Pinnately complex leaves are lustrous green and short deciduous. Early spring brings the arrival of fall, which is swiftly followed by the arrival of new foliage. Pea-like clusters of white, pink, or purple fragments bloom. Pongam is a fast-growing evergreen tree that can reach a height and spread of 40 feet. It is one of the few trees that can fix nitrogen. Leaves are pinnately complex up to three inches in length and are momentarily deciduous in the spring, dropping for a brief period before being widely replaced by new foliage [2-4]. Tables 2 and 3.
The ‘Pongam Tree' stands with the crimson-colored cover for a week in March and April when the process of wilting lime-colored leaves is formed. The ‘Pongam tree' is becoming one of India's most admired city trees, being grown in a significant number of gardens and along endless highways. It grows wild in coastal forests around India, as well as alongside streams and rivers. The buds on the Pongamia tree turn scarlet in March and April as they mature. There are five white petals in addition to those that are pink or violet in color. Fruits are woody pods that expand in length. Seeds are protected by a sturdy raft that resembles a rubber ship. Oil is produced in 30-40% of seeds. They grow to a dark grey color just before fresh leaves sprout. The ‘Pongam tree' has five, seven, or nine oval-shaped leaflets with pointy points on its leaves. The leaves are 15 cm to 30 cm long and have a short stalk. The roots of flower and leaf stalks are generally plump [5, 6]. This drought-tolerant, nitrogen-fixing plant can endure water logging, moderate cold, and salinity. It has a high tolerance for arid climates and low soil moisture levels. In tea plantations, it is used as a wind break and shade tree. It has the potential to be used to make biodiesel. P. pinnata is known for its therapeutic characteristics and has been utilized in traditional medicine. Medicinal properties can be found in almost every section of the plant [7]. (Fig. 1).
| Plant Type | Medium-sized evergreen, perennial, and deciduous trees Height: 35 to 40 feet Stem Diameter: 50-80cm Growth Rate: Fast Texture: Medium Chromosome Number: 22 |
|---|---|
| Growth Requirements | Light requirement: - the tree grows in full sun. Soil tolerances: - clay; loam; sandy; slightly alkaline; acidic; well-drained. Drought tolerance: high Aerosol salt tolerance: moderate Winter interest: no special winter |
| Care and Prunin | All parts of the plant are toxic and will induce nausea and vomiting if eaten. |
3. CHEMICAL COMPOSITION OF PONGAMIA PINNATA
Six substances (two sterols, three sterol derivatives, and one disaccharide) are found in P. pinnata seeds, together with 8 fatty acids (three saturated and five unsaturated). The presence of metabolites such as galactoside, sitosteryl acetate, stigmasterol, galactoside, and sucrose was the first to be recorded; the number of saturated and unsaturated fatty acids (two monoenoic, one dienoic, and two trienoic) were the same [8].
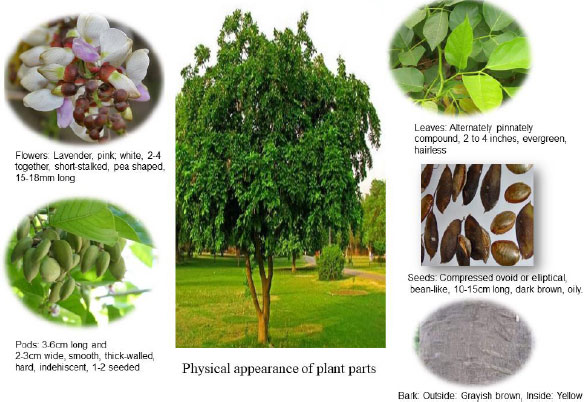 |
Fig. (1). The physical appearance of plant parts. |
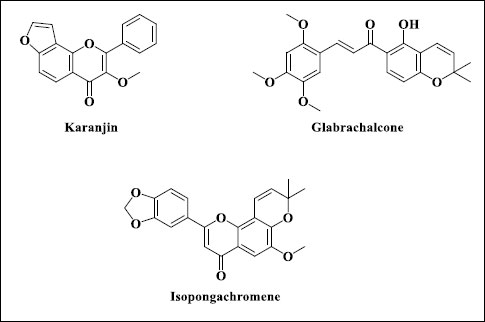 |
Fig. (2). Phytochemicals from Pongamol pinnata [9]. |
Karanjin, pinnatin, pongagalabrone, pongamol, pongapin, and kanjone have been isolated from seeds. A 'pongol' derivative of flavone is present in immature seeds. Glabrachalcone and isopongachromene are two more flavonoids that have been isolated from the seeds (Fig. 2).
Six compounds (two sterols, three sterols) are found in the seeds of Pongamia pinnata. Pongone, Galbone, Pongalabol, Pongagallone A, and B are just a few of the flavone and chalcone derivatives found in the plant's leaves and stem. Spectrophotometric analysis was used to deduce their structure, which was then compared to one previously published. In vitro, tests were performed on Pongamones A-E against DHBV RCs DNAP and HIV-1 RT [9]. (Fig. 3).
3.1. Properties and Action (Ayurvedic)
P. pinnata commonly known as ‘Karanj’ has been used widely in traditional medicinal practice for a long time. It contains various phytoconstituents belonging to alkaloids, glycosides, flavonoids, fixed oils, and carbohydrates. The root paste is applied in scrofulous enlargement. Due to its anti-helminthic property, it is used in treating, ophthalmology, dermatopathy, vaginopathy, and ulcers [10].
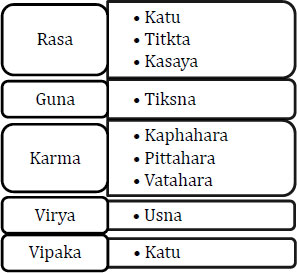 |
Fig. (3). Use of P. Pinnata in Ayurvedic medicine. |
4. PHYTOCHEMISTRY OF PONGAMIA PINNATA
Different sections of P. pinnata can be used to isolate a variety of chemicals. Many are flavonoid derivatives (flavones, flavans, and chalcones), as well as a variety of other chemicals (terpenes, steroids, and fatty acids).
Every plant portion has a specific application, and the whole plant has several medical characteristics. Following the separation of flavonoid derivatives, phytochemical research on P. pinnata is carried out. P. pinnata chemicals have low toxicity to mammalian cells, indicating that they are safe to employ. The first compound isolated was karanjin, furanoflavonol which is also a trade mark compound of the species found especially in India and China [3]. Pongamia has long been utilized as a folk medicinal plant, particularly in the Indian medicine systems of Ayurveda and Siddha [11].
Current Science was the first to report on the discovery of a novel crystalline substance derived from Pongamia glabra oil. Combustion analysis revealed that the chemical's molecular formula is C9H7O2, and molecular weight investigations revealed that the molecular weight just needs to be doubled, as validated by the analysis of the methoxy compound. Pongamol may also be extracted from press cake by solvent extraction. Different solvents with increasing polarity were used in a series of extractions. In comparison to less polar solvents, more polar solvents produced a higher yield. This indicated that polar chemicals were identified in greater abundance in the twigs of the P. pinnata plant than non-polar compounds [12]. Physical investigation and preliminary phytochemical screening of P. pinnata ethanolic extract, such as qualitative chemical analysis and HPTLC, reveal the existence of twelve distinct peaks, confirming the presence of twelve chemicals [13, 14]. (Fig. 4).
5. BIOLOGICAL ACTIVITY OF PONGAMIA PINNATA
Flavones, chalcones, isoflavones, flavanones, and pterocarpanoids have all been discovered because of phytochemical research on this plant. Pterocarpanoids are the second most abundant class of natural isoflavonoids and are major phytoalexins. Antioxidant, anti-inflammatory, anticancer, and antimalarial properties are all found in pterocarpanoids. They all appear to have broad-spectrum activities such as antioxidant, antibacterial, anti-inflammatory, and anti-diabetic properties. Many more phytochemical substances, including flavonoids and terpenoids, have been discovered, and their investigation has shown a wide range of biological functions. Antioxidant, anti-parasite, antimicrobial, anti-inflammatory, anti-diabetic, anti-convulsant, anti-hyperammonemia, anthelminthic, insecticidal, cytotoxic, and immunomodulatory actions are among these activities.
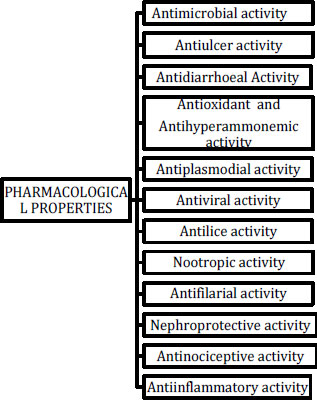 |
Fig. (4). Biological activities exhibited by Pongamia Pinnata. |
All plant parts are utilized as a cure for cleaning filthy ulcers, gum, and tooth difficulties in gonorrhea, and local scrofulous enlargement in one form or another. The flavor of fresh bark is pleasant and mucilaginous, but it quickly turns bitter and caustic. Flowers are prescribed to treat glycosuria and diabetes. Leaf extracts have also been proven to be beneficial in the treatment of leprosy, dyspepsia, and cough. Ulcers and wounds are cleaned with a hot infusion. Oil is used in the manufacture of biodiesel. In addition to being used as a tonic and fever reducer, powdered seeds are also used to treat bronchitis and whooping cough [14, 15].
5.1. Anti-oxidant Activity
Cancer, inflammatory response syndrome, lung disorders, liver diseases, and atherosclerosis are all caused by free radicals and reactive oxygen species. Free radicals such as superoxide anions (O2), hydrogen peroxide (H2O2), and hydroxyl free radicals (OH) can easily react with molecular oxygen to generate species that damage biomembranes, proteins, and DNA. Lipid peroxidation is another prominent and harmful impact of free radicals that have been connected to a variety of disorders2. The chain reaction of these peroxides can result in the creation of malondialdehyde, a more stable compound (MDA). Malondialdehyde is a three-carbon molecule that is one of the most common secondary breakdown products of polyunsaturated fatty acids (PUFA). MDA is known to be cytotoxic, and it has been detected at high levels in a variety of illnesses linked to free radical damage. Flavonoids and tannins are strong antioxidants that can be found in almost every component of a plant. P. pinnata leaf extract has antioxidant and circulatory lipid peroxidation action [16].
Pongamones, furanoflavones, pongamol, pongagalabrone, and pongapin, pinnatin, and kanjone are potent antioxidants. The extracts of P. pinnata have high free radical scavenging activity, which can be attributed to the flavones found in the extracts. As a result, plants may be effective in the treatment of diseases caused by free radicals.
The antioxidant activity could be attributed to the extract's phenolics, which have strong radical-scavenging properties. The redox characteristics of phenolics, which allow them to operate as reducing agents, hydrogen donors, and singlet oxygen quenchers, are primarily responsible for their activity [17].
5.2. Anti-bacterial Activity
As we confront the major problem of various drug-resistant infections and the development of novel microbiological agents is very expensive, the use of medicinal plants as a source of alternative antibiotics has become an essential component of science [18]. Pongamia pinnata is used for bronchitis, whooping cough, scabies, leprosy, piles, ulcer, and rheumatic joint pain [19]. When tested on Staphylococcus aureus, E. coli, Candida albicans, E. aerogenes, P. aeruginosa, S. pyogenes, S. typhi, A. niger, S. epidermidis, and Micrococcus leteus, the methanolic extract of P. pinnata showed considerable microbial inhibitory characteristics, according to Deepak et al. The oil produced from P. pinnata was found to have antibacterial action against Aspergillus niger, Staphylococcus aureus, and Pseudomonas aeruginosa when tested using the Minimum Inhibitory Concentration (MIC) method and the dry weight method [12]. Mycobacterium and skin infections are also resistant to the plant extract. Using the disc diffusion method, the anticarcinogenic activity of P. pinnata twigs was tested against the dental caries-causing bacteria S. mutans, S. sobrinus, L. casei, and A. odontolyticus [20].
The extract contains a variety of phytochemicals and exhibits strong antibacterial activity against S. aureus and K. pneumoniae. Protein leaking from the membrane has been proposed as a mechanism of action for this function. The third of the five bands seen on TLC exhibited antibacterial action. The presence of various functional bands in this band was established using infrared spectroscopy and UV-Vis spectroscopy [20]. The extract contains a variety of phytochemicals and has strong antibacterial properties against S. aureus and K. pneumoniae. MIC tests on pathogens such as Aspergillus niger, Staphylococcus aureus, and Pseudomonas aeruginosa demonstrate that the plant inhibits its growth, implying that it has antibacterial properties. Its anti-diarrheal effect is highlighted by a decrease in the amount of cholera toxin and bacterial penetration of epithelial cells. As a result, significant antibacterial and anti-fungal properties were discovered.
Aqueous extracts of the seed coat of P. pinnata (Linn.) Pierre was evaluated against methicillin-resistant Staphylococcus aureus in conjunction with different medications. P. pinnata was synergistic with ampicillin, meropenem, cefazolin, cefotaxime, cefpirome, and cefuroxime in 70% to 100% of cases, with a fractional inhibitory concentration (FIC) index of 0.5. Except for aztreonam, practically all of the combinations demonstrated synergistic effects when using the time-kill approach with 0.5 minimum inhibitory concentration (MIC) of P. pinnata in conjunction with 8, 4, 2, and 1 g mL-1 of the various other antibiotics.
5.3. Antifungal Activity
Several studies have investigated the antifungal activity of P. pinnata against various fungal strains. One study found that the methanolic extract of P. pinnata had significant antifungal activity against Candida albicans, a common fungal species that can cause infections in humans. The study also found that the extract had higher antifungal activity than the standard antifungal drug fluconazole. Another study investigated the antifungal activity of P. pinnata against dermatophyte fungi, which are known to cause skin infections. The study found that the ethanolic extract of P. pinnata had significant antifungal activity against dermatophyte fungi such as Trichophyton mentagrophytes and Microsporum canis. Furthermore, a study conducted on the antifungal activity of P. pinnata against Aspergillus flavus and Aspergillus niger found that the plant extract had a significant inhibitory effect on the growth of these fungal species. It was also found that the antifungal activity of P. pinnata was attributed to the presence of flavonoids and tannins. Overall, the available evidence suggests that P. pinnata has significant antifungal activity against various fungal species. Further research is needed to fully elucidate the mechanisms underlying this activity and to determine the potential use of P. pinnata as a natural antifungal agent [21].
5.4. Anti-alzheimer Activity
In developed countries, neurodegenerative illnesses are becoming a major concern among the elderly and workers. Today's lifestyle causes oxidative stress, which is a prevalent cause of neurodegenerative illnesses. Alzheimer's disease is a form of dementia that affects memory, thinking, and behavior. Because this disease is mostly associated with memory and behavior problems, scientists investigated the possibility of karanjin and embelin in restoring memory deficiencies in one study. In a dose and time-dependent manner, both isolated compounds and the standard effectively restored diazepam-induced amnesia and improved learning and memory in mice [22]. Recent studies have explored the potential of P. pinnata in treating various neurodegenerative disorders, including Alzheimer's disease.
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is characterized by the accumulation of amyloid-β plaques and the hyperphosphorylation of tau protein in the brain. These pathological changes lead to cognitive impairment, which is the hallmark of AD. The potential of P. pinnata as an anti-Alzheimer's agent is attributed to the presence of various phytoconstituents, including flavonoids, terpenoids, steroids, alkaloids, and tannins. These compounds are known to possess potent antioxidant and anti-inflammatory properties that can counteract the pathophysiological processes of AD [23].
5.5. Anti-diabetic Activity
P. pinnata has been traditionally used for medicinal purposes, including the treatment of diabetes. Several studies have investigated the antidiabetic activity of P. pinnata and its potential use as a natural remedy for diabetes. One study investigated the effect of P. pinnata leaf extract on blood glucose levels in diabetic rats. The study found that the extract significantly reduced blood glucose levels in diabetic rats compared to a control group. The researchers suggested that the antidiabetic activity of P. pinnata may be due to its ability to increase insulin secretion and improve glucose uptake. Another study investigated the effect of P. pinnata seed extract on blood glucose levels in diabetic rats. The study found that the extract significantly reduced blood glucose levels and improved insulin sensitivity compared to a control group. The researchers suggested that the antidiabetic activity of P. pinnata may be due to its ability to increase insulin sensitivity and improve glucose metabolism. Furthermore, a study conducted on the antidiabetic activity of P. pinnata in humans found that the plant extract significantly reduced fasting blood glucose levels in diabetic patients. The study also found that the extract improved insulin sensitivity and reduced the levels of glycated hemoglobin, which is a marker of long-term blood glucose control. The antidiabetic activity of P. pinnata is attributed to the presence of various bioactive compounds such as flavonoids, alkaloids, and terpenoids. These compounds have been shown to have insulin-like effects and to improve glucose metabolism in animal and human studies. Additionally, the herb is utilized to treat hypertension and hyperlipidemia8. In STZ-induced diabetic rats and genetically diabetic db/db mice, the antihyperglycemic activity of the pure chemicals pongamol and karanjin extracted from the chloroform-soluble fraction of the ethanolic extract of P. pinnata fruit was examined. Both drugs had significant glucose-reducing action, according to the findings [3]. Pongamol administration increased glucose transport and GLUT4 translocation to the cell surface in L6-GLUT4myc myotubes in a concentration-dependent manner, without affecting the total quantity of GLUT4 protein or GLUT4 mRNA, effects that were additive with insulin. Insulin-mediated phosphorylation of AKT was dramatically potentiated by pongamol (Ser-473). For diabetic individuals, P. pinnata extract therapy may be a safe alternative to anti-hyperglycemic drugs. For diabetic individuals, P. pinnata extract might be used as a secure anti-hyperglycemic treatment [24].
Overall, the available evidence suggests that P. pinnata has significant antidiabetic activity and may be a potential natural remedy for diabetes. However, further research is needed to fully elucidate the mechanisms underlying this activity and to determine the optimal dosage and duration of treatment.
5.6. Wound Healing Properties
The destroyed tissue's organization must be restored systematically. Inflammation, proliferation, and remodelling of the damaged tissue are all stages of wound healing. Homeostasis causes inflammation, and vasoconstriction of blood vessels causes the release of inflammation mediators. The proliferation phase includes fibroblast granulation and angiogenesis, which results in improved collagen fibers. Eicosanoids, prostaglandins, leukotrienes, and reactive oxygen species are also released during healing (ROS).In Wistar rats, a reduction in wound area was noted 21 days after surgery. P. pinnata 's early wound healing is supported by increased wound contraction and tensile strength, increased hydroxyproline and hexosamine concentration, antioxidative activity, and moderate antibacterial activity. One of the ways for speeding wound healing may be the induction of cytokine production. P. pinnata may be effective in the tropical management of wound healing, according to the findings [17].
5.7. Anti-cancer Activity
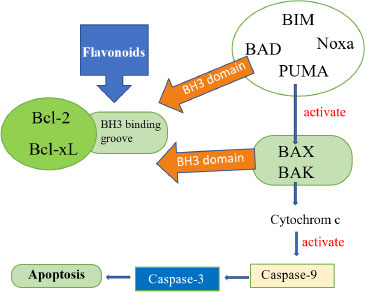
Breast cancer is the 2nd leading cause of death in women after lung cancer. MCF-7 is a frequently utilized epithelial cancer cell line derived from breast adenocarcinoma for in vitro breast cancer investigations because it retains some desirable properties of the mammary epithelium [30]. Under normal circumstances, cytochrome P450-1A1 (CYP1A1) expression is inhibited, but it is activated in the majority of breast tumours by polycyclic aromatic hydrocarbons (PAHs), which can be converted by CYP1A1 to carcinogens. As CYP1A1 inhibitors, phytochemicals or dietary flavonoids may aid in the prevention of PAH-mediated carcinogenesis and breast cancer [25]. The anticancer activity of P. pinnata seed extract-fabricated zinc oxide nanoparticles (Pp-ZnO NPs) on human MCF-7 breast cancer cells can be observed [26]. At dosages more than 50 mg/ml, cytotoxicity experiments demonstrated that a single treatment with Pp-ZnO NPs dramatically reduced the cell survival of breast cancer MCF-7 cells. Phase-contrast microscopy was used to investigate morphological changes in MCF-7 breast cancer cells treated with Pp-ZnO NPs. In CYP1A1-overexpressing normal human HEK293 cells, a methanolic extract of seeds from P. pinnata inhibits CYP1A1 quite efficiently. Its secondary metabolites, the furano-flavonoids pongapin/lanceolatin B, have an IC50 of 20 nM for inhibiting CYP1A1. The G0-G1 phase of the cell cycle of CYP1A1-overexpressing MCF-7 breast cancer cells is also blocked by methanolic extract of P. pinnata seeds, which represses cyclin D1 levels and induces cellular senescence [27]. The chemicals differentially inhibit the growth of different cancer cell lines (most effective on HeLa cells), with very minimal inhibitory effect on the growth of normal mouse embryonic fibroblast cell lines. It was discovered that karanjin might reduce intracellular reactive oxygen species (ROS) by inhibiting IB breakdown, which resulted in NFB nuclear translocation being restricted [22]. Pongapin and Plumbagin boosted DNA damage-induced p53 and p21 nuclear expression considerably. Karanjin therapy, on the other hand, resulted in little DNA damage and enhanced p53 expression. The chemicals caused G2/M arrest and a rise in the SubG1 population, indicating apoptotic induction. The substances also caused caspase-dependent apoptosis by altering the Bax/BCl2 ratio, either by increasing Bax expression by Pongapin and Plumbagin or by lowering BCl2 expression by Karanjin. Pongapin and Karanjin, like Plumbagin, could be promising natural anticancer drugs in the future [28].
Pongapin and Karanjin, two Furano flavonoid derivatives, were compared to Plumbagin, a plant-derived polyphenol having anticancer action [29]. The antitumor effect of lonchocarpin from traditional herbal medicine P. pinnata was studied. Using fresh leaves extract of P. pinnata gold nanoparticles (AuNPs) were synthesized. Cytotoxic effects of AuNPs against human cervical cancer cell line (HeLa) were also investigated [15]. Another basic work employing P. pinnata leaf extract to synthesize gold nanoparticles revealed antitumor efficacy. Breast cancer cell line (MCF-7) growth was suppressed by biogenic gold nanoparticles with an IC50 of 1.85 g/mL. Another basic work employing P. pinnata leaf extract to synthesize gold nanoparticles revealed antitumor efficacy. Breast cancer cell line (MCF-7) growth was suppressed by biogenic gold nanoparticles.
Lonchocarpin inhibited cell growth by altering the Bax/Caspase-9/Caspase-3 signaling pathway. An apoptotic test utilizing flow cytometry revealed that lonchocarpin caused 41.1 percent and 47.9 percent apoptosis, respectively, after 24 and 48 hours of treatment. Furthermore, at doses of 25, 50, and 100 mg/kg, lonchocarpin suppressed tumor growth in S180-bearing mice, with inhibition rates of 57.94, 63.40, and 72.51 percent, respectively. These findings suggested that lonchocarpin could be effective as a natural cancer therapy agent [30] (Fig. 5).
5.8. Anti-inflammatory Activity
P. pinnata, a highly valued plant of the legume family, is widely used in traditional medicine for its anti-inflammatory properties. The active components of the plant are derived from the leaves, seeds, and bark, which possess potent anti-inflammatory, antioxidant, and antimicrobial effects. The anti-inflammatory activity of P. pinnata is attributed to the presence of various phytoconstituents, such as flavonoids, terpenoids, steroids, alkaloids, and tannins. These constituents act by inhibiting the activity of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6, and by modulating the action of enzymes such as cyclooxygenase (COX) and lipoxygenase (LOX). Studies have shown that the leaf extract of P. pinnata inhibits the production of nitric oxide (NO) and prostaglandin E2 (PGE2) in LPS-stimulated RAW264.7 macrophage cells, a model of acute inflammation. The inhibition of NO production is attributed to the suppression of iNOS gene expression, while the inhibition of PGE2 production is due to the downregulation of COX-2 expression. These findings suggest that P. pinnata exerts its anti-inflammatory effects by inhibiting the activity of pro-inflammatory enzymes and cytokines in macrophages. Another study investigated the anti-inflammatory effects of P. pinnata seed oil in a model of chronic inflammation induced by formalin injection in rats. The oil was found to significantly reduce both the paw volume and the levels of pro-inflammatory cytokines such as TNF-α and IL-6. These effects were attributed to the presence of flavonoids and terpenoids in the seed oil, which has been shown to possess potent anti-inflammatory properties. In addition to its anti-inflammatory effects, P. pinnata also possesses potent antimicrobial effects against a wide range of pathogens. This property makes it a valuable addition to the treatment of infectious diseases that often involve inflammation. Overall, the anti-inflammatory activity of P. pinnata is due to the presence of various phytoconstituents that act by inhibiting pro-inflammatory cytokines and enzymes. The plant extract has been shown to possess potent anti-inflammatory and antimicrobial effects, making it a promising candidate for the development of natural remedies for inflammatory and infectious diseases [31].
5.9. Anti-ulcer Activity
Several studies have investigated the anti-ulcer activity of P. pinnata and its potential use as a natural remedy for ulcers. One study investigated the effect of P. pinnata leaf extract on gastric ulcers in rats. The study found that the extract significantly reduced the formation of gastric ulcers and improved the healing of existing ulcers compared to a control group. The researchers suggested that the anti-ulcer activity of P. pinnata may be due to its ability to increase the production of mucus in the stomach, which protects the stomach lining from damage. Another study investigated the effect of P. pinnata seed extract on gastric ulcers in rats. The study found that the extract significantly reduced the formation of gastric ulcers and improved the healing of existing ulcers compared to a control group. The researchers suggested that the anti-ulcer activity of P. pinnata may be due to its ability to inhibit the production of gastric acid and increase the production of mucus in the stomach. Furthermore, a study conducted on the anti-ulcer activity of P. pinnata in humans found that the plant extract significantly reduced the severity of gastric ulcers and improved the healing of existing ulcers. The study also found that the extract reduced the levels of inflammatory markers in the stomach lining, which suggests that it has anti-inflammatory activity. The anti-ulcer activity of P. pinnata is attributed to the presence of various bioactive compounds such as flavonoids, alkaloids, and tannins. These compounds have been shown to have anti-inflammatory and antioxidant effects, which may contribute to the anti-ulcer activity of P. pinnata. In acetylsalicylic acid (ASA) ulcerated rats, there was a decrease in gastric juice volume, acid output, and peptic activity but no effect on mucin activity. In contrast to the offensive acid pepsin production, it was shown that it increases mucosal defence components such as mucin secretion, mucosal cell life span, mucosal cell glycoproteins, cell proliferation, and lipid peroxidation prevention [16]. Overall, the available evidence suggests that P. pinnata has significant anti-ulcer activity and may be a potential natural remedy for ulcers. However, further research is needed to fully elucidate the mechanisms underlying this activity and to determine the optimal dosage and duration of treatment [32].
5.10. Anti-lice Activity
The head louse pediculus humanus capiti was tested with various extracts of P. pinnata leaves. The results demonstrated that P. E. extract has anti-pediculicidal properties, while methanol extract has mild pediculocidal properties. The foundation for research into novel anti-lice medicines from medicinal plants was formed by patterns of increasing drug resistance to pediculicidal treatments for head louse infestations. P. pinnata leaf extracts were evaluated in the study against the head louse pediculus humanus capitis [33].
5.11. Nootropic Activity
Nootropics are a class of compounds that enhance cognitive function, including memory, creativity, and motivation. P. pinnata is an herbal plant that has been traditionally used in Ayurvedic medicine for a variety of medicinal purposes, including enhancing cognitive function. Recent research indicates that P. pinnata has nootropic activity that can potentially improve cognitive function. P. pinnata seed extracts decreased pentobarbitone sleeping time in rats probably by stimulation of the hepatic microsomal enzyme system (a). Petroleum ether extract (PEE) of its roots enhanced sleeping time by suppressing the central nervous system (CNS) [34].
5.12. Anti Filarial Potential
P. pinnata is a medicinal plant with various pharmacological properties, including anti-filarial potential. Several studies have demonstrated the potential of P. pinnata as an effective anti-filarial agent. In one study, the ethanolic extract of P. pinnata was evaluated for its anti-filarial activity against Brugiamalayi, a filarial parasite that causes lymphatic filariasis in humans. The results showed that the extract exhibited significant activity against the parasite and inhibited its motility and viability. Another study evaluated the anti-filarial potential of P. pinnata seed oil and its major component, karanjin, against the human filarial parasite Wuchereriabancrofti. The oil and karanjin showed significant activity against the parasite and inhibited its growth and viability. The aqueous and alcohol extracts of fruits and alcohol extracts of leaves show ant filarial potential on cattle filarial parasites. It inhibits the whole worm's natural movement as well as the S. cervi nerve-muscle preparation. Overall, these studies suggest that P. pinnata has promising anti-filarial potential and can be further explored for the development of novel therapeutics for filarial infections [35].
5.13. Nephroprotective Activity
P. pinnata is a medicinal plant that has long been used for various therapeutic purposes, including nephroprotective activity. Several studies have demonstrated the potential of P. pinnata as an effective nephroprotective agent. One study investigated the protective effect of P. pinnata leaf extract on kidney damage induced by cisplatin, a chemotherapeutic agent known to cause nephrotoxicity. The results showed that the extract significantly reduced the levels of kidney damage biomarkers and improved kidney function compared to the untreated group. Additionally, the extract exhibited antioxidant and anti-inflammatory activities, which are believed to contribute to its nephroprotective effects. Another study evaluated the nephroprotective potential of P. pinnata flower extract against gentamicin-induced nephrotoxicity in rats. The results indicated that the extract significantly reduced the levels of kidney damage biomarkers and improved kidney function compared to the untreated group. The extract also exhibited significant antioxidant activity. A recent study investigated the protective effects of P. pinnata bark extract on kidney damage induced by lead toxicity in rats. The results showed that the extract significantly reduced the levels of kidney damage biomarkers and improved kidney function, indicating its nephroprotective potential. The extract also exhibited significant antioxidant and anti-inflammatory activities. Overall, these studies suggest that P. pinnata has promising nephroprotective potential and can be further explored for the development of novel therapeutics for kidney-related disorders [36].
5.14. Antinociceptive Activity
Pongamia pinnata, also known as the Indian beech, is a medicinal plant that has been used for a variety of therapeutic purposes, including antinociceptive activity. Several studies have demonstrated the potential of P. pinnata as an effective antinociceptive agent. One study examined the antinociceptive activity of P. pinnata leaf extract in mice using the hot plate and writhe tests. The results showed that the extract significantly increased the pain threshold and reduced writhing behavior compared to the control group, indicating its antinociceptive potential. The study also suggested that the extract may be acting through opioid and non-opioid mechanisms. Another study evaluated the antinociceptive effects of P. pinnata bark extract in rats using the acetic acid-induced writhing test and the formalin-induced paw-licking test. The results showed that the extract significantly reduced the number of writhes and paw-licking time, indicating its potential as an antinociceptive agent. The study suggested that the extract may be acting through the inhibition of prostaglandin synthesis and modulation of opioid receptors.
A recent study investigated the potential antinociceptive activity of P. pinnata stem bark extract in mice using the hot plate test and acetic acid-induced writhing test. The results showed that the extract exhibited significant antinociceptive activity in both tests, indicating its potential as a natural analgesic. The study also suggested that the extract may be acting through the inhibition of cyclooxygenase-2 and activation of opioid receptors. Overall, these studies suggest that P. pinnata has promising antinociceptive potential and can be further explored for the development of novel natural analgesics [37].
5.14.1. Skin Care
P. pinnata oil is used in many topical creams and ointments for treating skin ailments. It is used to reduce skin irritation, itchiness, and inflammation. The oil has also been shown to reduce the appearance of scars and improve skin texture by increasing collagen and elastin production. Its antibacterial and antifungal properties help in reducing skin infections. P. pinnata extracts have been shown to exhibit antimicrobial activity against the bacteria that cause acne, such as Propionibacterium acnes. A study published in the Journal of Applied Microbiology in 2020 demonstrated that P. pinnata leaf extract inhibited the growth of P. acnes and reduced inflammation in human skin cells. P. pinnata oil is rich in fatty acids and emollients, which can help moisturize and hydrate the skin. A recent study published in the Journal of Natural Products in 2021 investigated the composition and biological activity of P. pinnata seed oil. The study found that the oil contained significant amounts of linoleic acid and oleic acid, which can improve skin barrier function and hydration. Overall, P. pinnata has promising skincare benefits that can be further explored for cosmetic and medicinal applications [38].
5.15. Insecticidal Properties
The plant is known to have insecticidal activity, which makes it useful in controlling insects and pests. Here are some recent references detailing the insecticidal activity of Pongamia pinnata:
Insecticidal activity against mosquitoes: A study published in the Journal of Insect Science, in 2021, reported the insecticidal activity of P. pinnata seed extract against Aedes aegypti and Anopheles stephensi mosquitoes. The extract exhibited significant larvicidal and pupicidal activity against both mosquitoes and showed no toxicity against non-target organisms.
Insecticidal activity against agricultural pests: A study published in the Archives of Phytopathology and Plant Protection in 2020 reported the insecticidal activity of P. pinnata seed extract against three major agricultural pests, i.e., Helicoverpa armigera, Spodoptera littoralis, and Myzuspersicae. The extract exhibited significant insecticidal activity against these pests, making it a potential natural insecticidal agent for controlling agricultural pests.
Insecticidal activity against stored product pests: A study published in the Journal of Stored Products Research in 2021 reported the insecticidal activity of P. pinnata leaf extract against two stored product pests, i.e., Tribolium castaneum and Sitophilus oryzae. The extract showed significant insecticidal activity against these pests and can be used as an alternative to synthetic insecticides for the control of stored product pests
Mechanism of insecticidal action: A study published in the Journal of Asia-Pacific Entomology in 2021 investigated the mechanism of insecticidal action of P. pinnata extracts against two agricultural pests, i.e., Spodoptera litura and Helicoverpa armigera. The study showed that the extract disrupted the cellular membrane integrity and caused oxidative stress in the pests, leading to their death.
Formulation development: A recent study published in the Journal of Plant Protection Research in 2021 reported the development of a P. pinnata seed oil-based formulation for the control of the diamond back moth, Plutella xylostella. The formulation exhibited significant insecticidal activity against the pest and can be a potential alternative to synthetic insecticides.
Overall, the insecticidal activity of P. pinnata is promising, and further research is needed to fully explore its potential in insect pest management [39-42].
5.15.1. Memory
Studies have shown that P. pinnata has a significant effect on improving spatial memory and learning. The plant extracts have been shown to improve memory retention and consolidation by enhancing the production of acetylcholine in the brain. The phytochemicals present in the plant such as flavonoids, catechins, and terpenoids are responsible for its memory-enhancing properties [43].
5.16. Anti-anxiety
P. pinnata has been found to have anti-anxiety effects. The plant extracts have been reported to have anxiolytic properties, which can potentially reduce anxiety and stress levels. The isoflavonoids and other bioactive compounds in P. pinnata have been shown to act as selective serotonin reuptake inhibitors (SSRIs), which are commonly used to treat anxiety disorders [44].
5.12. Neuroprotection
P. pinnata has also been found to have neuroprotective effects. The plant extracts have been shown to protect the brain against oxidative stress-induced damage, which can lead to cognitive impairment. The active compounds present in the herb, such as flavonoids and terpenoids, have antioxidant properties that protect the brain from damage caused by harmful free radicals. A study published in the Journal of Ethnopharmacology in 2021 investigated the neuroprotective activity of P. pinnata against neurotoxicity induced by amyloid-beta and glutamate in rat hippocampal cells. The findings suggested that P. pinnata extract exhibited significant protective effects against neuronal damage, indicating its potential as a natural neuroprotective agent [45].
Another study published in the Journal of Food Science and Technology in 2021 evaluated the protective effects of P. pinnata against oxidative stress-induced damage in human glioblastoma cells. The results showed that the extract exhibited potential protective effects on brain cells by stimulating antioxidant defences and reducing oxidative stress and cellular damage. A study published in the Indian Journal of Pharmaceutical Sciences in 2021 investigated the neuroprotective potential of P. pinnata leaves in rats exposed to neurotoxic stress. The findings suggested that the leaf extract exhibited significant neuroprotective effects by reducing oxidative stress and inflammation in the brain. Overall, these recent studies provide promising evidence for the neuroprotective effects of P. pinnata. However, more rigorous clinical trials are required to confirm its efficacy in human subjects [46].
P. pinnata has been found to have antidepressant effects. The phytochemicals present in the plant extracts have been shown to act as serotonin-norepinephrine reuptake inhibitors (SNRIs). SNRIs are a class of drugs commonly used to treat depression, anxiety, and other mood disorders. A study published in the International Journal of Pharmacognosy and Phytochemical Research in 2021 investigated the antidepressant potential of P. pinnata using a forced swim test in mice. The results suggested that the extract showed a possible antidepressant effect and could be useful in the management of depression. Another study published in the International Journal of Pharma and Bio Sciences in 2020 evaluated the antidepressant-like activity of P. pinnata in rats. The findings suggested that the ethanol extract of P. pinnata exhibited significant antidepressant-like activity by modulating neurotransmitter levels in the brain. Overall, these recent studies provide promising evidence for the antidepressant effects of P. pinnata. However, more rigorous clinical trials are required to confirm its efficacy in human subjects.
5.18. Other Properties
5.18.1. Pongamia as Biodiesel
Energy self-sufficiency is critical for a country's overall economic development. Considering the uncertain supplies and frequent price spikes of fossil fuels on the worldwide market, the necessity to find alternative energy sources that are sustainable, safe, and non-polluting takes precedence. Biodiesel (fatty acid methyl ester), a renewable, biodegradable, and non-toxic fuel generated from triglycerides through transesterification, has received a lot of interest in the last decade [47].
Pongamia seeds have a high oil content of up to 40%. It is quickly becoming the focus of several biodiesel research studies due to its ability to flourish on starved soils with low nitrogen and high salt levels. They yield higher-quality oil than other crops, encouraging non-edible fuel sources. They also don't compete directly with current farms, allowing them to be cultivated on degraded or marginal ground. It may also fix the nitrogen from the soil, reducing the requirement for additional fertilizers. While there are clear benefits of using Pongamia for biodiesel, there are several factors to consider when dealing with the world's complex energy situation. P. pinnata has been identified as one of the most ideal species due to several positive characteristics such as its hardiness, high oil recovery, and oil quality, among others. Because this oil has a high acid value, we must decrease it using the esterification and transesterification processes. This method of producing methyl ester yields a satisfactory result [10]. Pongamia is a legume tree with sees containing oils and fatty acids suitable for biodiesel production. It is currently being researched by the ARC Centre of Excellence for Integrative Legume Research as a feedstock for the biodiesel industry Table 4.
| Parts of Plants | Economical Values | Medicinal Values |
|---|---|---|
| Root | ● Fish Poison |
● Juice of roots with coconut milk and lime water used for gonorrhea ● Cleaning gums, Tooth ulcers ● Antihelmintic hence used in vaginal and skin diseases. ● cleansing foul ulcers and closing fistulous sore |
| Stem |
● Used as stove fuels and ornamental cravings ● Ash for dyeing purposes ● Agricultural implements, tool handles, and combs |
● Aqueous extract act as a CNS sedative and antipyretic activity |
| Leaf |
● Cattle fodder ● Insect repellent in grains ● Manure in rice |
● Juice is used for cold, cough, diarrhea, dyspepsia, flatulence, gonorrhea, leprosy ● antihelmintic, and digestive, and laxatives are used for inflammations, piles, and wounds. ● Relieve rheumatism treats itches and herpes. |
| Fruit | ● Fruits are edible |
● Used abdominal tumors ● Useful in ailments of the female genital tract, leprosy, tumor, piles, ulcers, and upward movement of the wind in the abdomen |
| Seed |
● Oil extraction is used as ‘green manure’ rich in protein and nitrogen ● Used as an insecticide |
● Used in keloid tumors, hypertension, skin ailments, and rheumatic arthritis ● The powder is valued as a febrifuge, tonic, and in bronchitis and whooping cough ● Anti-inflammatory, pectoral diseases, chronic fevers, hemorrhoids, and anemia |
| Oil |
● The oil is used for cooking fuel. Used as a lubricant, water-paint binder, pesticide, soap-making, candles, and tanning agent in industries ● Used as lipids for commercial processes ● Used in Cosmetics |
● Styptic, anthelmintic, and good in leprosy, piles, ulcers, chronic fever, and liver pain ● Used in rheumatoid arthritis, scabies ● Oil and zinc oxide are used for eczema |
| Bark |
● String and Rope made from bark fiber ● Paper pulp |
● For bleeding piles, for beriberi, reduce swelling of the spleen ● Useful in mental disorders, cough, and cold |
| Flower |
● Good source of pollen for honeybees ● Flowers are edible |
● Quench dips in diabetes ● Alleviating Vata and Kapha ● Used bleeding piles |
5.19. Toxicity
Some researchers investigated the toxicity of this plant. The ethanolic extract of the leaves did not exhibit any toxicity or mortality up to a dose level of 10.125 g/kg in the examination of the anti-inflammatory action in rats. Administration of the extracts (100, 300, 1000 mg/kg, p.o.) both acute and chronic did not produce any gastric lesions (Srinivasan et al. (2001)31. Through an anti-hyperammonemia study, Essa et al., 2005 reported that there was no significant difference in the weight of experimental animals as compared to controls (treatment without extract and ammonium chloride) [47]. Acute and chronic administration of methanolic extract of the stem bark to rats (200,500, and 1000 mg/kg p.o.) did not produce any gastric lesions. In mice, this extract did not show any sign of toxicity and mortality up to a dosage of 101.25 mg/kg p.o (Sagar et al., 2010) [35]. Aneela et al., 2011 also found that the administration of 2000 mg/kg crude seed extract to the rats was safe [48]. Gupta and Upadhaya 2017 conducted a study on mice to check the acute toxicity of crude seed suspension (powder) in mice and rats. 1600 mg/kg powder was administered orally for 20 days. The test drug did not produce any significant changes in organs however liver and spleen showed some mild to moderate changes. All hematological parameters were non-significant except hemoglobin which was highly significant as compared to the control group. So, caution should be exercised when this drug is administered in liver patients for longer durations [49].
In another study conducted by Jaiswal et al., 2011 toxic effect of the chemical constituent “karanjin” were also studied. They found that treatment of L6-GLUT4myc myotubes with karanjin caused a substantial increase in glucose uptake and GLUT translocation to the cell surface, in a concentration dependant manner, without affecting the total amount of GLUT mRNA and GLUT protein. Karanjin did not affect or alter the expression of key molecules in the insulin signaling cascade and did not affect the phosphorylation of AKT (Ser-473) [50].
Belagihally et al., 2011 also conducted a study to check the gastroprotective property of the chemical constituent “karanjin”.Karanjin when administered at a dose of 20mg/kg /oral for 14 days consecutively did not cause any lethal effects. There was no significant difference in alkaline phosphatase, serum glutamate oxaloacetate transaminases, serum glutamate pyruvate transaminase, and total protein between normal and karanjin-treated rats. It also not causes any significant change in body weight and food and water intake [51].
“Pongamol” another chemical compound of P. pinnata was studied for its toxicity by Bakiet al., 2007. When compared to rats in the control group, the administration of this drug at a dose of 300 mg/rat/day for 14 days straight did not result in any appreciable changes in body weight, biochemical, or hematological parameters. When comparing the experimental group to the control group, histopathological examination of the kidney, liver, heart, and lung revealed no abnormalities [52-55]. This research showed that this plant has remarkably low toxicity, which suggests that it may have potential as a treatment.
6. CURRENT STATUS, FUTURE PROSPECTS, AND CHALLENGES
Pongamia is a plant with several uses, yet its biodiesel-producing abilities are what make it more valuable. Commercial P. pinnata cultivation and production of biodiesel and bioethanol have not yet begun in Bangladesh. Pongamia and other fuel crops are not yet being cultivated on a large scale or in a commercial manner. In the lowest parts of Bangladesh's Sunamganj district, Pongamia trees are planted to prevent soil erosion and act as a windbreak. Koroch was cultivated naturally in Bangladesh at Ratargul Swamp Forest, Gowainghat, Sylhet. To improve rural poverty and emancipation, the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Andhra Pradesh, India, is funding creative research on the “journey from forest to micro-enterprise” of the Pogamia. This organization's goals are to restore damaged lands, protect the environment, evaluate and advance sustainable crop management techniques, and evaluate how planting Pongamia could increase the income of self-help organizations. After oil extraction, another goal is the value addition of its byproducts. About 28 institutions are working together in India to conduct joint research on a variety of topics, including the marking of superior planting materials, the evaluation of seed resources, the collection and storage of seeds, the phonological and chemical characterization of seeds, the improvement of trees to obtain high-quality and reliable seed sources, multi-location trials of superior planting materials, and the development of effective intercropping systems of tree-borne oilseeds. In South-eastern Queensland, Australia, it has been grown outside of its natural habitat.
The government, NGOs, and private-public partnerships should implement a major plantation program through commercial or social afforestation to meet future biodiesel demand and boost Pogamia's output. Participating local communities might not be very engaged until they understand the worth of plant genetic resources. Through a program to educate them about the economic viability and feasibility of cultivation as a choice on degraded lands and community wastelands, as well as by field demonstration, it will be ensured that the local population will be involved. Therefore, adding value to a plant would be an effective way to manage and propagate it in Pongamia while also emphasizing the way of life of the local communities. Field workers involved in Pongamia cultivation extension activities should receive training on the various facets of Pongamia plant cultivation, including the value of superior planting elements, gathering elite germplasm, developing nurseries, establishing plantations, and post-cultivation care. To improve the planting of Pongamia on more marginal wastelands, clonal cuttings of the elite materials or candidate plus trees may be made available. Early-bearing Pongamia types should be developed using various improved plant breeding techniques to shorten the growing time and boost oil content with acceptable oil characteristics, particularly monounsaturated fatty acid from the point of using it as biofuel. Again, choosing day-neutral verities is required to yield Pongamia seeds all year long. Therefore, initiatives for the development of dwarf high-yielding varieties that can minimize the management cost could also be considered. High-yield Pongamia types are produced in the “off-season”. To expand the range of growth and cultivation of Pongamia, further methods for creating location-specific genotypes resistant to challenging growing conditions such as salt, drought, alkalinity, and waterlogging can be studied.
CONCLUSION
P. pinnata has been widely employed as a therapeutic agent in ancient Ayurvedic medicine for several diseases. Concentrated fruits or seeds extract can be found in a range of herbal medications on the market today. Rheumatism is treated using P. pinnata preparation oil, which is readily available and used by natural healers. The P. pinnata plant is used for anti-hyperglycaemic, anti-inflammatory, anti-nociceptive, anti-plasmodial, anti-lipid peroxidative, anti-diarrhoeal, anti-ulcer, anti-hyper ammonic, antioxidant, and antibacterial purposes in traditional medical systems such as Ayurveda and Unani. Its oil is used to make biodiesel and also has an alternative energy source that is renewable, safe, and non-polluting. However, further study is needed to understand the mechanism of action of various chemical constituents exhibiting above mentioned biological activities. Moreover, further clinical research is needed on the toxicity of other compounds/chemicals isolated from this plant to ensure their eligibility to be used as a source of modern drugs.
LIST OF ABBREVIATIONS
| ICRISAT | = International Crops Research Institute for the Semi-Arid Tropics |
| GLUT | = Glucose transporter |
| CNS | = central nervous system |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Praveen Kumar Sharma is the Editorial Advisory Board Member for The Open Medicinal Chemistry Journal.
ACKNOWLEDGEMENTS
Declared none.