All published articles of this journal are available on ScienceDirect.
Microwave-Assisted Syntheses of Amino Acid Ester Substituted Benzoic Acid Amides: Potential Inhibitors of Human CD81-Receptor HCV-E2 In-teraction
Abstract
Results from our group showed benzyl salicylate to be a moderate inhibitor of the CD81-LEL–HCV-E2 interaction. To increase the biological activity, heterocyclic substituted benzoic acids were coupled to amino acid esters via microwave assisted DCC-reaction. The prepared compounds were tested for their inhibitory potency by means of a fluorescence labeled antibody assay system using HUH7.5 cells.
1. INTRODUCTION
Recently the World Health Organization (WHO) estimated that 3% of the world’s population has been infected with the Hepatitis C Virus (HCV) [1]. Infection with HCV is the most common cause of chronic hepatitis with frequent progression to liver cirrhosis and its sequelae [2]. Inhibition of the Hepatitis C Virus E2 glycoprotein (HCV-E2) binding to the largeextracellular loop (LEL) of the human cell surface protein CD81, a member of the tetraspanin family, prevents infection of human hepatocytes, the HCV principal target cells [3]. The aim of the present work was to prepare compounds which bind to CD81-LEL and therefore inhibit the CD81-LEL–HCV-E2 interaction.
This work was based on results recently achieved in our group [4]. Briefly, we found benzyl salicylate (Fig. 1) to inhibit the CD81-LEL–HCV-E2 interaction (25% at 50 µM) as outcome of a virtual screening followed by biological testing. Several heterocy-clic-substituted benzoic acid amides were synthesized and tested for their biological activity. Some of the prepared compounds showed inhibition of the CD81-LEL–HCV-E2 interaction.
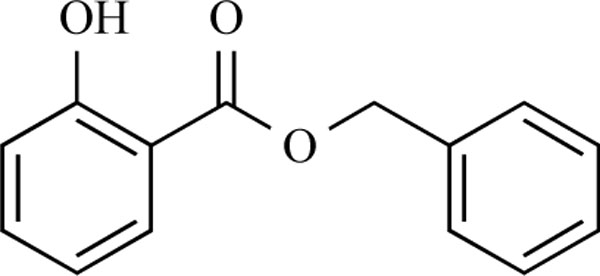
Benzyl salicylate.
2. RESULTS AND DISCUSSION
2.1. Structure Modification
Based on the docking pose of benzyl salicylate into the published x-ray structure of CD81-LEL (pdb code: 1IV5) [5], we assumed that an extension of the planar/aromatic ring system could increase the affinity to our intended binding site within a superficial cleft formed by two alpha-helices in the CD81-LEL structure. In order to maximize more specific hydrogen-bonding and electrostatic interactions with the target protein, we decided to introduce heterocyclic ring systems and amino acid moieties in opposite positions of an aromatic core structure. The aim of this work was therefore to prepare compounds of the general structure given in Fig. (2) and to determine their inhibition of the CD81-LEL–HCV-E2 interaction.
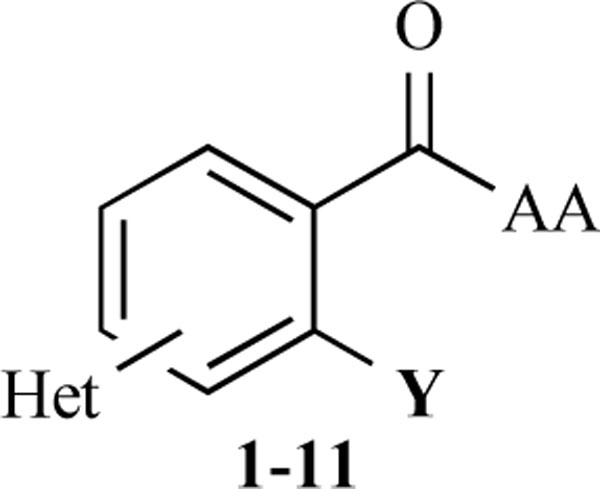
General structure of the synthesized benzoic acid amides (Het = Heterocycle, Y = -H, -OH, AA = L-alanine ethyl ester, Lphenylalanine ethyl ester, L-tryptophane methyl ester).
2.2. Syntheses and Biological Testing
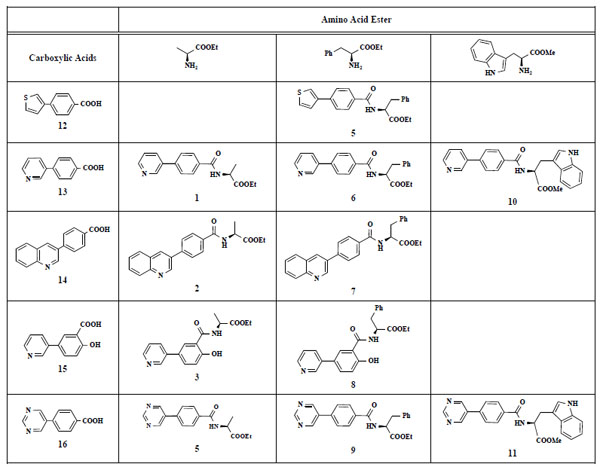
In the first step of the preparation, starting from commercially available reagents, the bromo substituted aromatic heterocycles were coupled to the correspon-ding boronic acids with tetrakis(triphenylphosphine) palladium (0) as catalyst via a Suzuki coupling reaction (cf. Scheme 1) in satisfying yields. The synthesized heterocyclic substituted carboxylic acids 12-16 as well as the applied bromo substituted heterocycles and boronic acids are shown in Table 1.
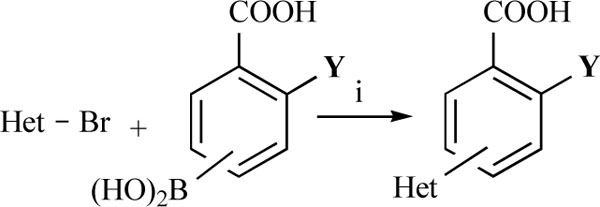
Reagents and conditions: (i) 10 ml EtOH + 15 ml 10% Na2CO3-solution, Pd(PPh3)4, O2-free, 900C over night (Y = -H, -OH)
Suzuki Coupling Reactions of Heterocycles and Boronic Acids Leading to Heterocyclic Substituted Carboxylic Acids (12-16)
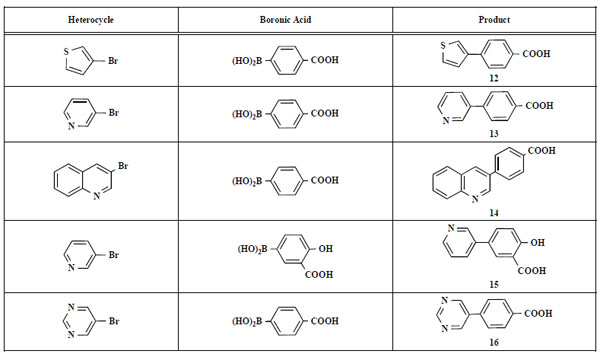 |
The desired amides 1-11 (Table 2) were obtained in the second step by connecting the benzoic acids 12-16 to L-alanine ethyl ester, L-phenylalanine ethyl ester and L-tryptophane methyl ester using N,N’-dicyclohexyl carbodiimide (DCC) as coupling agent for microwave-assisted amide formation (cf. Scheme 2).
Amino Acid Esters, Carboxylic Acids and the Corresponding ‘Combinatorial’ Target Compounds 1-11
 |
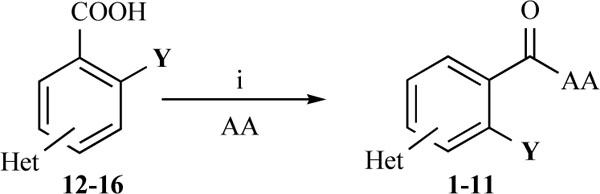
Reagents and conditions: (i) Dicyclohexyl carbodiimide, dimethoxyethane / dichloromethane (5/1), microwaves: 225 Watt, 13ºC, 5 bar, 10 minutes. Het = Heterocycle, AA = amino acid ester: L-alanine ethyl ester, L-phenylalanine ethyl ester, L-tryptophane methyl ester, Y = -H, -OH.
Before we decided to apply microwave-assisted amide syntheses, activation of the carboxylic acids by means of thionyl chloride followed by addition of the corresponding amino acid esters was tried. This did not lead to the desired compounds.
DCC coupling reaction under standard conditions was performed next leading to the desired amides in very poor yields. Therefore microwave-assisted DCC coupling reaction was tried to increase the yield of the desired products. This attempt finally led to the target compounds 1-11 in satisfying yields.
A medium throughput assay developed in our group [6] was used to test the prepared compounds 1-11 for their biological activity. This assay is based on the procedure of Pileri et al. [7] in which the inhibition of the interaction of the fluorescence-labeled CD81 antibody JS81 with HUH7.5 cells caused by our compounds is determined by FACS. The synthesized compounds 1-11 showed no increased inhibition concerning the CD81-LEL–HCV-E2 interaction compared to the original hit compound benzyl salicylate.
3. EXPERIMENTAL SECTION
3.1. General Procedure for the Suzuki Coupling Reaction
The boronic acid (1 equivalent) and the bromo substituted heterocycle (1 equivalent) were added to a mixture of 10 mL ethanol and 15 mL sodium carbonate solution (10%). This solution was free from oxygen by evacuating and flushing with nitrogen several times. After addition of 4 mol% of tetrakis(triphenylphosphine)palladium (0) the mixture was stirred at 90°C over night.
The remaining solid was filtered off at that temperature. Subsequently half of the solvent was removed under vacuum. The product precipitated after acidifying to pH 2 using formic acid. It was filtered off and dried under high vacuum.
3.2. General Procedure for the Formation of the Amides
The carboxylic acid was stirred with an equivalent amount of the amino acid ester and dicyclohexyl carbodiimide (1.2 equivalent) in the microwave oven (225 Watt, 135°C, 5 bar, 10 minutes) using dry dichloromethane (4 mL) as solvent. After filtration the solvent was removed. Purification of the crude product was performed with column chromatography.
3.3. Biological Test
HUH7.5 cells (1 * 105) were incubated with 100 µ l of the potential inhibitor (50 µM + 1% DMSO) in 96 transwell plates for 10 minutes at room temperature. Next 4 µL of the fluorescence-labeled CD81 antibody JS81 and 21 µL of phosphate buffered saline buffer (PBS) were added and kept at room temperature for 10 minutes. After appending 125 µL of PBS the cell suspension was incubated in the dark for 5 hours followed by FACS analysis.
3.4. Chemistry
Solvents and reagents were used as received from commercial distributors without further purification. Anhydrous reactions were conducted under a nitrogen atmosphere. Proton and carbon NMR spectra were recorded at a Bruker AM 500. The proton NMR spectra were recorded at 500 MHz, the carbon NMR spectra at 125 MHz. Chemical shifts δ are reported in ppm units. Molecular mass was determined by liquid chromatography – tandem mass spectrometry (LC-MS/MS) using a TSQ Quantum from Thermo Finnigan equipped with an electro spray interface and connected to a Surveyor HPLC (Thermo Finnigan). Positive and negative ion mass spectra were recorded (mass range m/z 150–1500) in normal scan mode. Melting points were determined using a Stuart Scientific SMP3 melting point apparatus. IR measurements were performed on a Bruker Vector 33 at a frequency range from 4000-250 cm-1. Wave numbers υ are reported in cm-1. Flash chromatography using Merck silica gel 35/40–63/70. Microwave assisted syntheses was performed using a CEM DISCOVER microwave oven.
(S)-Ethyl 2-[4-(pyridin-3-yl)benzamide]propanoate (1). Yield 24%. mp 118 °C IR 3330, 2930, 2851, 2478, 1745, 1606, 1479, 1437, 1210, 1170 1H-NMR (CDCl3) 8.75–8.74 (1 H, m), 8.52–51 (1 H, m), 7.90–7.88 (3 H, m), 7.60 (1 H, d, J = 8.51), 7.40–7.37 (1 H, m), 7.29–7.27 (1 H, m), 4.72–4.70 (1 H, m), 4.21–4.17 (2 H, q, J = 7.25), 1.48 (3 H, d, J = 7.25), 1.24 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 173.38, 166.86, 148.53, 147.70, 140.75, 135.86, 134.97, 127.97, 127.20, 123.95, 61.70, 33.70, 18.13, 14.00 LC/MS-MS 299.11 (M+H+).
(S)-Ethyl 2-[4-(quinolin-3-yl)benzamido]propano-ate (2). Yield 29%. mp 142 °C IR 3356, 2985, 1742, 1638, 1532, 1439, 1205, 1172 1H-NMR (CDCl3) 9.35 (1 H, s), 8.81 (1 H, s), 8.25–8.20 (4 H, m), 8.14–8.09 (3 H, m), 7.99–7.94 (1 H, m), 7.85–7.80 (1 H, m), 4.84–4.77 (1 H, m), 4.42–4.38 (2 H, m), 1.72–1.69 (3 H, m), 1.47 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 174.42, 169.59, 150.22, 148.12, 141.95, 135.74, 134.85, 131.46, 129.72, 129.59, 129.54, 129.27, 128.99, 128.68, 128.45, 128.21, 62.41, 50.35, 17.28, 14.50 LC/MS-MS 349.05 (M+H+).
(S)-Ethyl 2-[4-hydroxy-3-(pyridin-3-yl)benzamide] propanoate (3). Yield 14%. IR 2926, 1738, 1642, 1541, 1472, 1376, 1294, 1205 1H-NMR (CDCl3) 9.01 (1 H, s), 8.67–8.66 (1 H, m), 8.40–8.39 (1 H, m), 8.29–8.27 (1 H, m), 7.91 (1 H, dd, J = 8.51), 7.84–7.80 (1 H, m), 7.70–7.67 (1 H, m), 7.24 (1 H, d, J = 8.51), 4.85–4.81 (1 H, m), 4.41–4.39 (2 H, m), 1.71 (3 H, d, J = 7.25), 1.45 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 174.31, 170.15, 161.51, 148.31, 147.98, 136.00, 133.44, 133.05, 129.48, 128.15, 125.43, 119.49, 117.49, 62.49, 50.02, 17.48, 14.47 LC/MS-MS 314.97 (M+H+).
S)-Ethyl 2-[4-(pyrimidin-5-yl)benzamido]propano-ate (4). Yield44%. mp 124 °C IR 2930, 2475, 1741, 1625, 1550, 1415, 1213, 1161 1H-NMR (d4-MeOH) 9.35 (1 H, s), 9.30 (2 H, s), 8.22 (2 H, d, J = 8.55), 8.02 (2 H, d, J = 8.56), 4.82–4.78 (1 H, m), 4.42–4.37 (2 H, m), 1.71 (3 H, d, J = 7.57), 1.46 (3 H, t, J = 7.25) 13C-NMR (d4-MeOH) 174.35, 169.36, 158.47, 156.30, 138.54, 135.71, 135.06, 129.66, 128.28, 62.41, 50.34, 17.25, 14.50 LC/MS-MS 300.02 (M+H+).
(S)-Ethyl 3-phenyl-2-[4-(thiophen-3-yl)benzamido] propanoate (5). Yield 43%. mp 160 °C IR 3345, 2997, 1748, 1634, 1517, 1178 1H-NMR (CDCl3) 7.76 (2 H, d, J = 8.51), 7.64 (2 H, d, J = 8.51), 7.54–7.53 (1 H, m), 7.42–7.41 (2 H, m), 7.31–7.25 (3 H, m), 7.17–7.15 (2 H, m), 5.11–5.07 (1 H, m), 4.23 (2 H, q, J = 6.94), 3.32–3.23 (2 H, m), 1.28 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 171.66, 166.38, 141.16, 139.07, 135.94, 132.32, 129.45, 128.58, 127.65, 127.14, 126.67, 126.47, 126.16, 121.61, 61.67, 53.56, 37.99, 14.17 LC/MS-MS 380.00 (M+H+).
(S)-Ethyl 3-phenyl-2-[[4-(pyridin-3-yl)benzamido] propanoate (6). Yield 11%. mp 170 °C IR 3331, 2929, 1735, 1628, 1537, 1311, 1197 1H-NMR (CDCl3) 8.86 (1 H, s), 8.64–8.63 (1 H, m), 7.90–7.88 (1 H, m), 7.84 (2 H, d, J = 8.51), 7.64 (2 H, d, J = 8.51), 7.40–7.39 (1 H, m), 7.32–7.30 (2 H, m), 7.19–7.15 (3 H, m), 6.66–6.64 (1 H, m), 5.30 (2 H, s), 5.11–5.07 (1 H, m), 4.25–4.21 (2 H, m), 1.31–1.28 (3 H, m) LC/MS-MS 375.10 (M+H+).
(S)-Ethyl 3-phenyl-2-[4-(quinolin-3-yl)benzamido] propanoate (7). Yield 35%. mp 77 °C IR 3324, 2929, 2851, 1741, 1626, 1572, 1529, 1208 1H-NMR (CDCl3) 9.18 (1 H, s), 8.34–8.33 (1 H, m), 8.16–8.14 (1 H, d, J = 8.51), 7.91–7.88 (3 H, m), 7.79–7.73 (3 H, m), 7.61–7.59 (1 H, m), 7.32–7.28 (3 H, m), 7.18–7.17 (2 H, m), 6.71–6.69 (1 H, m), 5.12–5.09 (1 H, m), 4.25 (2 H, q, J = 6.94), 3.36–3.25 (2 H, m), 1.29 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 171.61, 166.25, 149.50, 147.77, 135.89, 133.66, 133.45, 132.66, 129.86, 129.44, 129.31, 128.61, 128.12, 127.94, 127.59, 127.21, 61.73, 53.62, 25.63, 14.16 LC/MS-MS 425.13 (M+H+).
(S)-Ethyl 2-[2-hydroxy-5-(pyridin-3-yl)benzamido]-3-phenylpropanoate (8). Yield 16%. mp 176 °C IR 3325, 2928, 2851, 1744, 1625, 1572, 1244 1H-NMR (CDCl3) 12.04 (1 H, s), 8.67 (1 H, s), 8.51–8.50 (1 H, m), 7.71–7.70 (1 H, m), 7.55–7.53 (1 H, dd, J1 = 8.83, J2 = 2.19), 7.41–7.40 (1 H, m), 7.30–7.27 (1 H, m), 7.25–7.21 (3 H, m), 7.11–7.09 (2 H, m), 7.02 (1 H, d, J = 8.83), 6.97 (1 H, d, J = 7.25), 4.98–4.94 (1 H, m), 4.17 (2 H, q, J = 7.25), 3.25–3.17 (2 H, m), 1.22 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 171.33, 169.11, 148.26, 147.82, 135.55, 133.97, 133.19, 129.35, 128.72, 127.43, 119.41, 114.56, 61.96, 33.95, 24.94, 14.14 LC/MS-MS 391.05 (M+H+).
(S)-Ethyl 3-phenyl-2-[4-(pyrimidin-5-yl)benzamido]propanoate (9). Yield 32%. mp 129 °C IR 3269, 2928, 1738, 1652, 1530, 1414, 1349, 1192 1H-NMR (CDCl3) 9.24 (1 H, s), 8.97 (2 H, s), 7.88 (2 H, d, J = 8.51), 7.65 (2 H, d, J = 8.51), 7.32 (3 H, m), 7.16–7.14 (2 H, m), 6.67–6.66 (1 H, m), 5.10–5.07 (1 H, m), 4.24 (2 H, q, J = 7.25), 3.34–3.24 (2 H, m), 1.41 (3 H, t, J = 7.25) 13C-NMR (CDCl3) 171.55, 165.93, 158.06, 154.97, 137.55, 135.80, 134.42, 133.34, 129.41, 128.62, 128.16, 127.24, 61.79, 53.62, 37.90, 14.17 LC/MS-MS 376.08 (M+H+).
(S)-Methyl 3-(1H-indol-3-yl)-2-[4-(pyridin-3-yl) benzamido]propanoate (10). Yield 24%. mp 96 °C IR 3266, 1737, 1642, 1530, 1435, 1215 1H-NMR (CDCl3) 8.75 (1 H, s), 8.55 (1 H, s), 8.40 (1 H, s), 7.80–7.77 (1 H, m), 7.70 (2 H, d, J = 8.51), 7.49 (3 H, s), 7.31–7.27 (2 H, m), 7.12–7.09 (1 H, m), 7.03–7.00 (1 H, m), 6.95–6.94 (1 H, m), 6.70 (1 H, d, J = 7.57), 5.11–5.08 (1 H, m), 3.66 (3 H, s), 3.44–3.36 (2 H, m) 13C-NMR (CDCl3) 172.38, 166.40, 149.09, 148.23, 141.04, 136.21, 134.47, 133.40, 127.94, 127.71, 127.22, 122.91, 122.31, 119.75, 118.63, 111.40, 109.95, 53.60, 52.49, 27.64 LC/MS-MS 400.08 (M+H+).
(S)-Methyl 3-(1H-indol-3-yl)-2-[4-(pyrimidin-5-yl)benzamido]propanoate (11). Yield 14%. mp 101 °C IR 3298, 1736, 1646, 1532, 1415, 1344, 1215 1H-NMR (CDCl3) 9.23 (1 H, s), 8.95 (2 H, s), 8.26 (1 H, s), 7.81 (2 H, d, J = 8.20), 7.60–7.55 (3 H, m), 7.36 (1 H, d, J = 8.20), 7.21–7.18 (1 H, m), 7.10–7.08 (1 H, m), 7.03–7.02 (1 H, m), 6.76 (1 H, d, J = 7.57), 5.20–5.16 (1 H, m), 3.76 (3 H, s), 3.53–3.47 (2 H, m) 13C-NMR (CDCl3) 172.31, 166.11, 157.87, 154.94, 137.37, 136.16, 134.32, 133.38, 128.27, 127.71, 127.12, 127.00, 122.82, 122.41, 119.83, 118.62, 111.38, 109.99, 53.65, 52.55, 27.59 LC/MS-MS 400.98 (M+H+).
4-(Thiophen-3-yl)benzoic acid (12). Yield 94%. mp 280 °C (Lit. 281-282 °C) [8] 1H-NMR (d6-DMSO) 8.05–8.04 (1 H, m), 7.96 (2 H, d, J = 8.51), 7.85 (2 H, d, J = 8.20), 7.63–7.68 (1 H, m), 7.64–7.63 (1 H, m). 13C-NMR (d6-DMSO): 167.02, 140.31, 139.09, 129.92, 129.07, 127.46, 126.14, 125.99, 122.79.
4-(Pyridin-3-yl)benzoic acid (13). Yield 62%. mp 215 °C (Lit. 215 °C) [9] 1H-NMR (D2O/TFA) 7.80 (1 H, s), 7.61–7.56 (2 H, m), 7.00 (2 H, d, J = 8.51), 6.96–6.93 (1 H, m), 6.56 (2 H, d, J = 8.51) 13C-NMR was not applied due to the TFA.
4-(Chinolin-3-yl)benzoic acid (14). Yield 92%. 1H-NMR (d6-DMSO/TFA: 1/1) 9.66 (1 H, s), 9.51 (1 H, s), 7.80–7.78 (1 H, m), 8.38 (1 H, d, J = 7.88), 8.27 (1 H, d, J = 8.51), 8.15–8.08 (5 H, m), 7.98–7.95 (1 H, m) [Lit. 250 MHz, d6-DMSO: 13.09 (1 H, br s), 9.32 (1 H, d), 8.76 (1 H, d), 8.07 (5 H, m), 7.83 (2 H, m), 7.67 (1 H, m)] [10] 13C-NMR (d6-DMSO + TFA) 166.79, 144.39, 132.64, 131.15, 130.18, 127.61, 129.76, 129.47, 118.44, 116.15, 113.85, 111.56.
5-(Pyridin-3-yl)salicylic acid (15). Yield 46%. mp 260 °C (Lit. 263 °C) [11] 1H-NMR (D2O/TFA: 1/1) 7.61 (1 H, s), 7.42–7.40 (1 H, m), 7.36–7.35 (1 H, m), 6.93 (1 H, d, J = 2.52), 6.78–6.75 (1 H, m), 6.49 (1 H, dd, J = 8.51), 5.84 (1 H, d, J = 8.83) 13C-NMR was not applied due to the TFA.
4-(Pyrimidin-5-yl)benzoic acid (16). Yield 60%. mp 218 °C (Lit. 220 °C) [12] 1H-NMR (d6-DMSO) 9.17 (1 H, s), 9.15 (2 H, s), 8.01 (2 H, d, J = 8.20), 7.74 (2 H, d, J = 8.20) 13C-NMR (d6-DMSO) 169.60, 157.11, 154.60, 140.35, 133.92, 133.18, 131.47, 131.39, 129.85, 128.75, 125.81.


