All published articles of this journal are available on ScienceDirect.
Synthesis of Medicinally Important Quinazolines and Their Derivatives: A Review
Abstract
The study of biodynamic heterosystems has proved to be the most attractive and useful for the development of potential drugs with superior properties and works effectively for the treatment of a variety of diseases, including pandemic ones. Out of the thousand biodynamic heterosystems, the quinazoline heterosystem is one that exhibits wide-ranging biological and pharmacological properties. Synthesis of potential medicinal material is comparatively challenging and needed enough time for clinical trials, testing, permissions for concerned authorities, production and supply. Therefore, researchers usually focused on novel methods incorporated with a high yield of previously approved chemical compounds. This present review article has focused on the synthesis of medicinally important quinazolines and their derivatives, which produce reaction products in less time with high yields, including more attention with eco-friendly green synthesis approaches incorporating multicomponent reactions (MCR), ionic liquids and microwave irradiations.
1. INTRODUCTION
Human society in the present time experiences a variety of problems related to health, and few of them are in the category of a pandemic. Pandemic problems generally arise in a very short period of time and cause an unexpected adverse effect on human development. At this stage, researcher/chemist provides instant solutions. Researchers mainly adopt two approaches: either synthesis of potential medicinal material against said deceases or production of existing chemical material by adopting new methods, which replace time-consuming complex existing methods to avoid the shortage and make available. Synthesis of potential medicinal material is comparatively challenging and requires enough time for clinical trials, testing, permissions for concerned authorities, production and supply. Therefore, researchers usually focused on other synthetic methods of previously approved chemical compounds. For more than a century, in the first stage, chemists mainly found solutions to targeted chemicals in heterocyclic compounds. In this sequence, the synthesis of heterocyclic compounds has more concerns with heterocyclic chemistry along with pharmaceutical/medicinal chemistry. The formulation of biodynamic heterosystems by fusion has been verified to be a very smart and convenient way for scheming novel molecular structure of prospective medicinal material with variable pharmacological activities [1-28]. Heterocyclic compounds synthesis with fused hetero-systems includes, in many cases, a multistep synthetic approach, which needs a great number of synthetic procedures together with extraction and purification of every single step. Synthetic methodologies with multistep, consequently, headed to the synthetic ineffectiveness along with the production of huge amounts of waste. Hence, in view of chemistry with a sustainable approach [29], the development of new synthetic ways which provides selective admittance to clarified scaffolds combined with a molecular variety [30] and compatibility with the environment [31] has always been a countless competition for chemical and medicinal research.
Due to their in-operation effortlessness and capability to produce only one product from more than two components in a single synthetic process, multiple bond-forming efficiencies, and high atom economy [32], multicomponent reactions (MCRs) have arisen as the most effective synthetic approach [33]. In recent times, multicomponent reactions (MCRs) have been involved in medicinal-pharmaceutical research and appeared as synthetically most important reactions for the preparation of heterocyclic compounds, which can be used as potential drugs. The MCRs, including Biginelli, Hantzsch and Ugi condensation reactions, as well as a number of newly discovered MCRs, have been used for the synthesis of heterocycles(fused) with the introduction of structural diversity and molecular diversity.
Environmentally benign and cost-effective catalytic systems, used as important materials in the main themes of modern synthetic chemistry, were prepared by the use of the most effective principles of green chemistry. In this point of view, due to avoidable vapor pressure (vp) and non-flammable nature [34], Ionic liquids (ILs) have been used as alternatives to hazardous volatile organic solvents. Therefore, various organic chemists nowadays use ionic liquids (ILs) as catalysts/solvents [35].
In recent years, the use of ionic liquids (ILs) either as solvents or catalysts has shown significant changes in the results of many organic transformations. Ionic liquids (ILs) have shown superiority as solvents with many advantages over traditionally used organic solvents in synthetic organic reactions. It has been reported that the structure of the ionic liquid has an intense effect on the solubility, reactivity and other possible reaction variables. With a sensible grouping of cations and anions, it is promising to adjust the solvent behavior in order to modify the most suitable ionic liquid (IL) for synthetic applications. It has been observed that by the practice of ionic liquids (ILs), the yield of the final product can be optimized only with the minor change of the ions: anions or cations. Moreover, the combination of functional groups can improve their function by increasing most probably stability, suitability and reusability of ILs as compared to the non-functionalized equivalents [36].
Brønsted acidic ionic liquids-ILs (combine the advantages of solid acids and mineral acids) have been used as catalysts in many synthetic transformations (acid-catalyzed) and produced significant interest [37]. Bronsted acidic ionic liquids (ILs) and their homogeneous system can be readily separated from the reaction products along with miscible water. Thus, ILs can be used in both homogeneous and heterogeneous catalysis. Functionalized ionic liquids can be readily synthesized so that the ILs have advantages over already exiting conventional catalysts. Important ionic liquids (ILs) can be treated as effective catalysts and can be recovered without any appreciable loss in catalytic activity.
The presence of SO3H functional group in ionic liquids improves their solubility in water with superior acidities [38]. Thus, SO3H functional group-containing ionic liquids can be used as important acid catalysts in addition to their use as solvents so that the ionic liquids can be used as a green substitute catalyst to the existing conventional acid catalysts, such as HF, H2SO4, AlCl3,etc. in various chemical syntheses [39].
Due to the presence of quinazoline structure as a building block in various natural products and synthetic pharmaceuticals, researchers have attracted their attention towards the synthesis of quinazolines in view of their wide-ranging biological and therapeutic activities [40] including antibacterial [41], anti-fungal [42], anti-cancer [43-46], anti-inflammatory [44] and antihypertensive [45] activities. Furthermore, quinazoline derivatives have also been well-recognized for their use as anticonvulsants [47], sedatives [48], vasodilator [49-51], phosphodiesterase inhibitors [52], and fibrinogen receptor antagonists [53].3-Aminoquinazoline-2,4-diones (AQDs) have been reported as powerful topoisomerase inhibitors [54].
Quinazoline derivatives have recently been evaluated as calcitonin gene-related peptides [55, 56] and vasopressin V3 receptors [57]. Additionally, quinazolinones have also been reported to exhibit anti-proliferative activities and also shown inhibitory effects for thymidylate synthase, such as Doxazosin mesylate 1 with quinazolinone structural moiety is an alpha-blocker and nowadays being used for the treatment of high blood pressure and prostatic hyperplasia (BHP). Quinazoline derivatives 2 can also be used as strong inhibitors of the epidermal growth factor (EGF) receptors of tyrosine kinase (EGFR) routed through a realistic system with the binding of ATP [58] (Fig. 1).
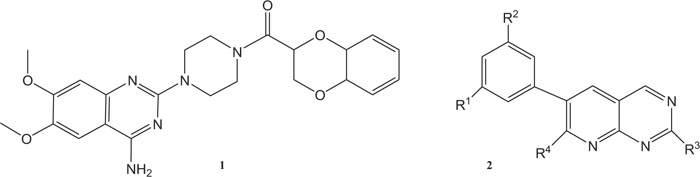
Quinazolines and their derivatives were employed as important ligands, especially for benzodiazepine and GABA receptors in the central nervous system [59] or as deoxyribonucleic acid DNA binders [60]. Quinazoline and their derivatives have also been used to increase lipid solubility [61]. Quinazoline derivatives have also been reported to exhibit antitubercular, antimalarial, antiviral, antimicrobial, anti-inflammatory and diuretic activities [62-64].
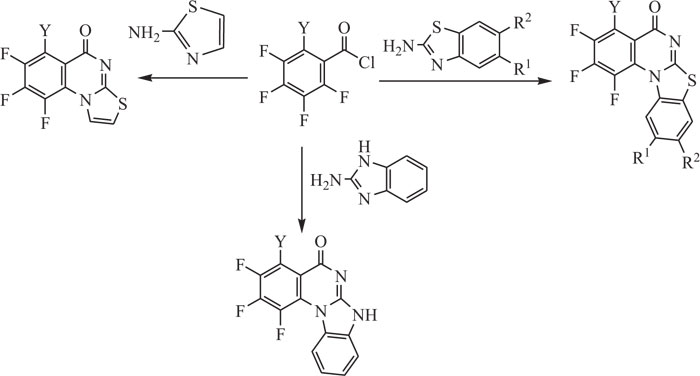
Due to their extraordinary behavior as bio-active material, quinazolines are elegant targets in organic synthesis. Quinazolines and their derivatives have been used as potent intermediates, especially for the preparation of clinical pharmaceuticals, drug intermediates, alkaloids and other materials. Quinazolinic structures [65] have been synthesized by different synthetic processes like Niementowski’s synthesis [66], Bischler’s synthesis [67] and Riedel’s synthesis [68-70]. Annulated fluroquinazolines were synthesized by the reaction of 2-aminobenzothiazole, 2-aminoimidazole and 2-aminothiazole with poly-fluorobenzoyl chlorides in the presence of di-phenyl ether (Scheme-1) [71]. The annulated fluoroquinazolines have been reported to exhibit wide-ranging biological activities such as antibacterial, antiviral, antitumour, etc. [72, 73].
Conventional methods of synthesis suffer from many disadvantages of organic synthesis, i.e., low yield, multistep procedure, time-consuming process, etc. Therefore, in view of the generation of structurally diverse molecules with structural complexity and interesting bio-activities in the least count of synthetic steps, annulated quinazolines have been synthesized by multicomponent reactions [74].
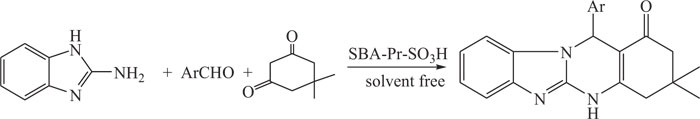
Quinazolinones derivatives such as benzimidazo-quinazolinones have been synthesized by a more convenient multicomponent reaction (MCR) of aromatic aldehydes with 2-aminobenzimidazole and dimedone using reusable sulfonic acid functionalized nano-porous silica (SBA-Pr-SO3H) under solvent-free conditions as more eco-friendly and nano solid acid catalyst (Scheme-2) [75].
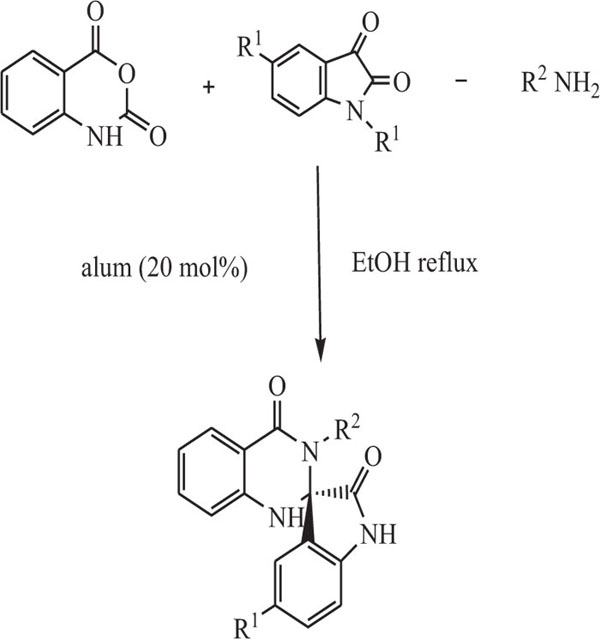
Mohammadi et al. [76] introduced an important way for the preparation of substituted quinazoline-(3’H)-diones involving cyclo-condensation of amines, isatins, isatoic anhydride, and green catalyst like KAl(SO4)2.12H2O (alum), (Scheme-3).
Zeng et al. [77] introduced a versatile and innovative path for the synthesis of fused quinazolines involving iodine catalyzed multicomponent reaction (MCR) of active methylene compounds with aromatic aldehydes (structurally diverse) and 5-aminotetrazole (Scheme-4).
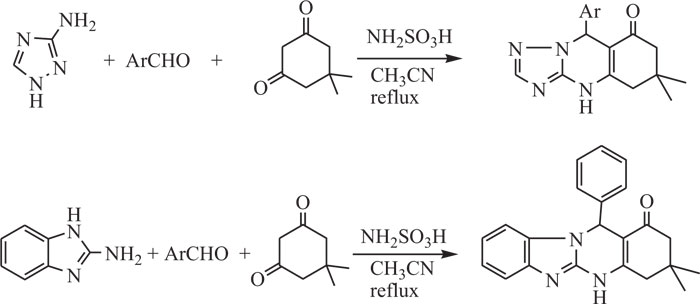
Recently, Heravi et al. [78] synthesized triazolo/ benzimidazoloquinazolinones by an easy and effective three-component, a one-pot process which includes condensation of amine sources like 3-amino-1,2,4-triazole/2-aminobenzimi- dazole with different aldehydes and dimedone in the presence of sulfamic acid as green catalyst in acetonitrile under thermal conditions (Scheme-5).
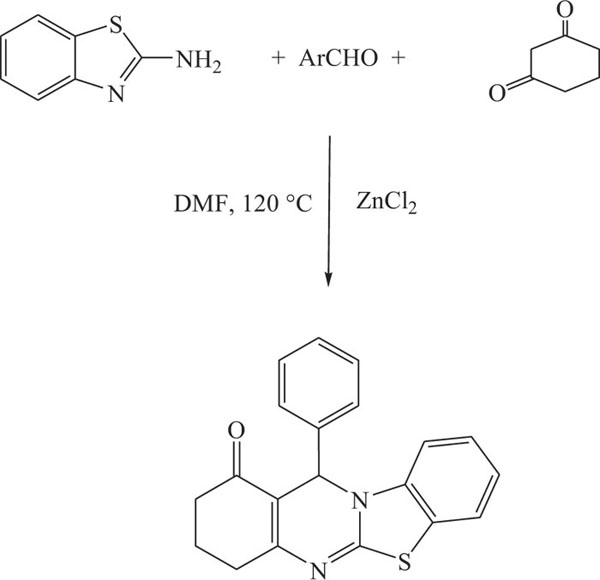
With the same approach, convenient synthesis of benzothiazoloquinazolin-1-ones has also been reported [79] in which process involved the reaction of substituted benzenethiols with aromatic aldehydes and cyclohexanedione in refluxing DMF using anhydrous zinc chloride as catalyst (Scheme-6).

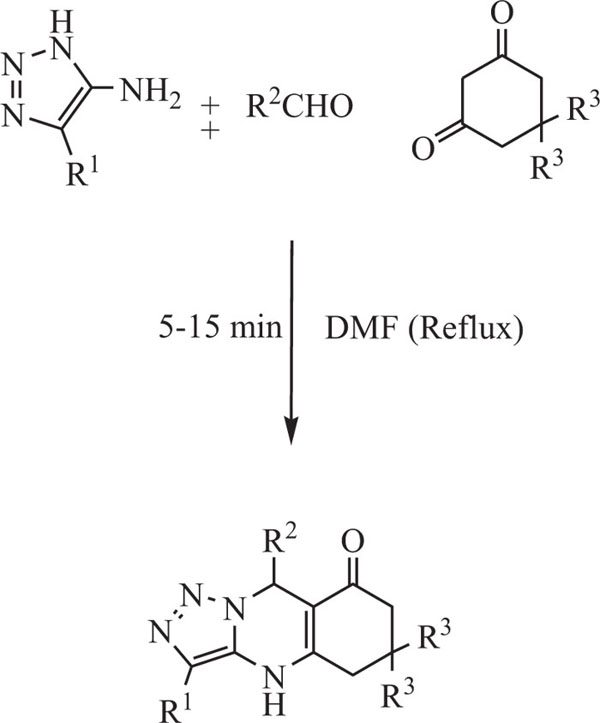
Gladkov et al. [80] recently synthesized triazoloqui- nazolin-8-ones by multicomponent reaction (MCR) of 4-amino-1,2,3-triazoles with cyclic diketones (1,3) and aldehydes(aromatic) in refluxing DMF (Scheme-7).
1,2,3,4-Tetrahydroquinazolines [81] were synthesized by the use of two different methods, grinding and oil bath. Both the methods involved the reaction of 2-aminobenzylamine and benzaldehydes and observed approximately the same yield. But oil bath method is most effective with respect to the required time. The reaction was also performed with water as a solvent and required more time as compared to both methods used earlier (Scheme-8a).
Fu et al. developed an efficient method for the synthesis of pyrazolo[1,5-c] quinazolines via a one-pot two-step process involving readily available substituted 1-(2- halophenyl)-3-alkylprop-2-yn-1-ones, hydrazine hydrochloride and amidine hydrochlorides using inexpensive CuI as a catalyst. The method affords a new strategy for the construction of diverse and useful poly N-heterocyclic compounds. (Scheme-8b)


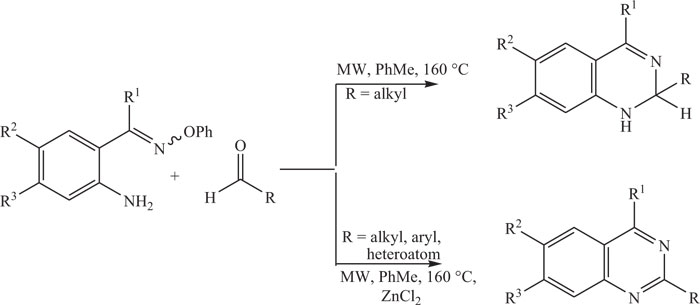
The practice of conventional solvents, i.e., tetrahydrofuran, toluene, dioxane, benzene and dimethyl formamide, can limit the attractiveness of these synthetic methods. Organic solvents used in conventional methods are often considered harmful to human health and responsible for adverse effects on the environment components, i.e., biotic and abiotic. Therefore, subjected to high waste disposal costs and government restrictions because of environmental concerns, researchers focused to develop new methodology incorporating microwave irradiations for the synthesis of important annulated quinazolines. Functionalized quinazolines were synthesized by microwave influenced reaction of O-phenyl oximes with aldehydes. 2-aminoarylalkanone O-phenyl oximes were synthesized and then treated with an aldehyde in toluene under microwave heating to obtain functionalized dihydro- quinazolines (Scheme-9) [82-84].
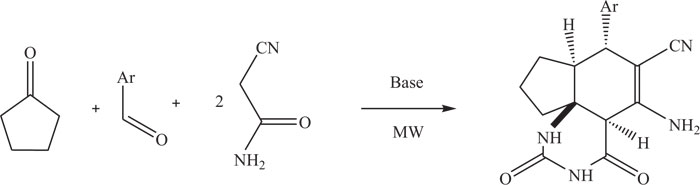
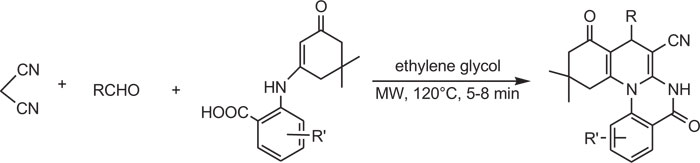
Substituted quinazoline-carbonitriles were synthesized by the multicomponent reaction of aldehydes, cycloketones, and cyanoamides, which act as both substrates and nucleophiles. Under microwave irradiation (M.W.), reaction was performed with ease by mixing four reactants and potassium carbonate in ethylene glycol (Scheme-10) [85].
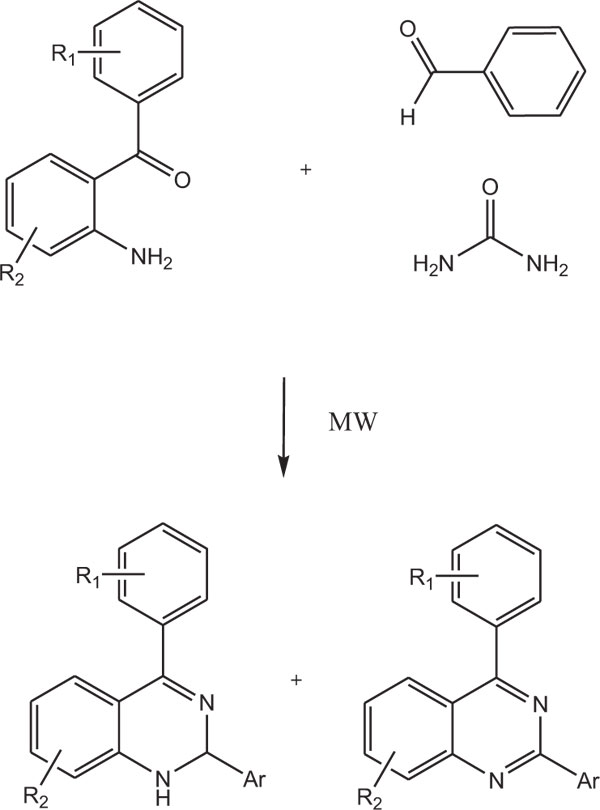
2,4-Disubstituted1,2-dihydroquinazolines were also synthesized by multicomponent reaction (MCR) of aromatic aldehydes and 2-aminobenzophenone under M.W. conditions using urea as a source of ammonia. In this reaction, the corresponding quinazoline is also obtained as a minor product (Scheme-11) [86].
Quinolinoquinzolines and other fused heterocycles have also been synthesized by the microwave-assisted multicomponent reaction of enaminones, malonitrile and aldehydes. In this synthesis, the lactam ring is generated as a result of the reaction (Scheme-12) [87].
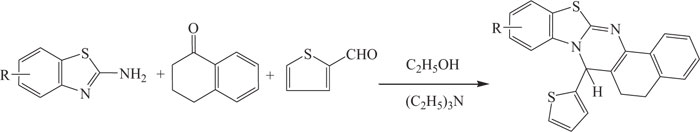
Kumar et al. introduced a one-pot, three-component sequential synthesis for the synthesis of benzothia- zoloquinazolines, incorporating medicinally privileged heterosystems (Scheme-13a)
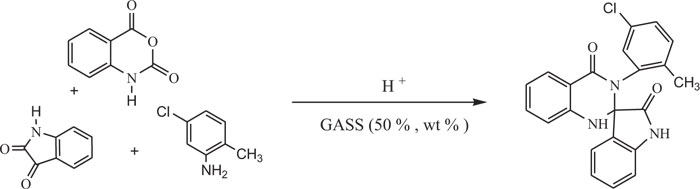
The literature survey reveals that bio-based solvents are suitable for the wide scope of organic reactions. These solvents hold considerable additional promise to reduce the environmental impact. Khandelwal et al. developed a convenient and environmentally benign diversity-oriented synthetic strategy to synthesize 2,3-dihydroquinazolin-4(1H) -one derivatives using GAAS as a bio-based green solvent. The synthesis of 2,3-dihydroquinazolinones involved a multicomponent reaction of isatoic anhydride, substituted anilines and aromatic aldehydes in gluconic acid aqueous solution (50%, wt %). (Scheme-13b) [88].
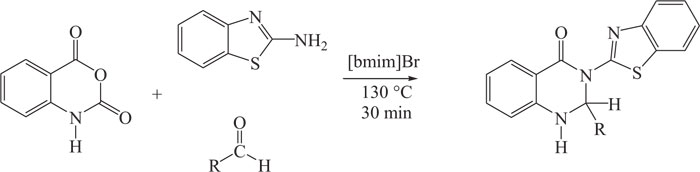
In the present time, the researchers have focused on the synthesis of quinazolines using ionic liquids (ILs) as eco-friendly solvents and catalysts. As compared to the conventional acid catalysts (homogeneous and heterogeneous), acidic ILs have demonstrated numerous advantages [89-96]. 2, 3-dihydroquinazolin-4(1H)-ones [97] were prepared by an efficient multicomponent reaction that involved the use of ionic liquids as a solvent-catalyst system. The synthetic method involved the reaction of 2-aminobenzothiazole with isatoic anhydride and different aldehydes in the presence of ionic liquids (Scheme-14).
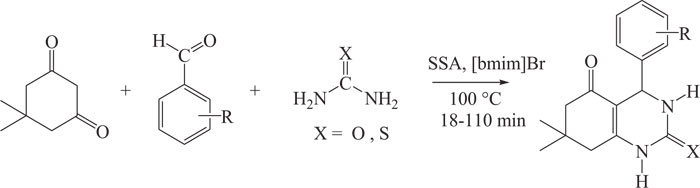
Quinazoline- 2,5-diones were prepared by the Biginelli reaction. The reaction involved the reaction between substituted dimedones, aldehydes and urea/thiourea using a combination of [bmim]Br and silica sulfuric acid as a catalyst (Scheme-15) [98].
4(3H)-Quinazolinones were synthesized by ionic liquid promoted synthetic method involving the reaction of 2-amino-benzamide with substituted aroyl chlorides in the presence ionic liquids like1-(4-sulfonic acid)butylpyridinium hydrogen sulfate [(CH2)4SO3HPY][HSO4], and 3-methyl-1-(4-sulfonic acid)butylimidazolium hydrogen sulfate [(CH2)4SO3HMIM] [HSO4] as green and reusable catalysts with an excellent yield of the reaction product (Scheme-16) [99].
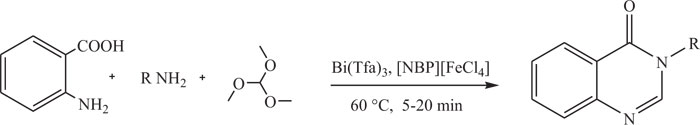
The reaction, reaction of anthranilic acid, trimethylorthoformate and primary amines in the presence of a catalytic amount of Bi (TFA)3 in ionic liquid butylpyridiniumtetrachloroferrate [NBP][FeCl4] also provided quinazolinones (Scheme-17) [100].
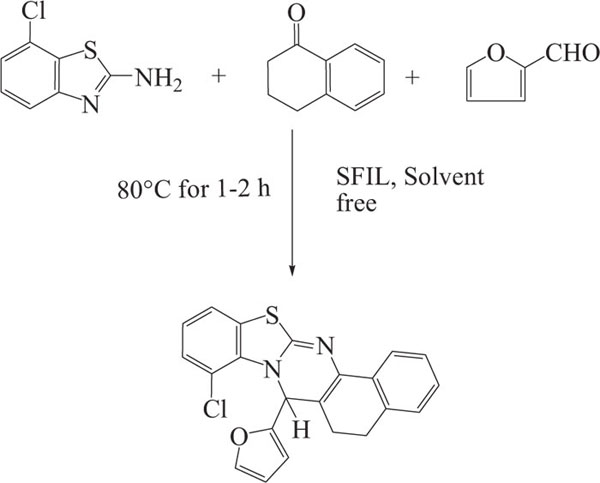
Sharma et al. reported the synthesis of benzo thiazoloquinazolines- an ecofriendly, efficient synthetic method which involved three-component domino reaction of 2-aminobenzothiazoles, a-tetralone, and furan-2-carbaldehyde/p-methoxybenzaldehyde using an SO3H-functionalized halogen-free ionic liquid ([MIM(CH2)4SO3H][HSO4]) as a catalyst and solvent a(Scheme-18) [101].
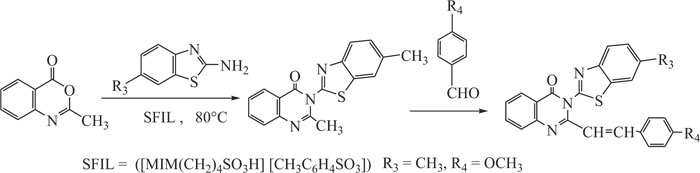
Kumar et al. developed a new synthetic approach for the synthesis of 3-benzothiazolyl-2-styrylquinazolin-4(3H)-ones with excellent yields using ionic liquid as catalyst and reaction medium. The present method provided excellent results as compared to the results observed with the use of conventional solvents such as DMF, CH3CN, glacial acetic acid, and ethanol (Scheme-19) [102-104].
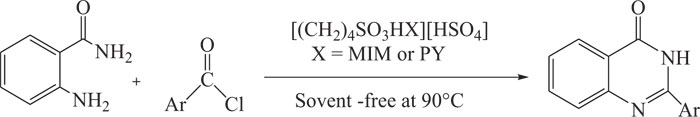
CONCLUSION
The literature survey reveals that very few publications have appeared in the literature related to the synthesis of quinazolines and their derivatives, which could not provide sufficient information to the researchers engaged in the synthesis of quinazolines and related drug-like small molecules incorporating quinazoline molecule. Most of the publications appeared in the literature are based on the synthesis of quinazolines using conventional methods. The present review article includes recent developments in the synthesis of quinazolines with the emphasis particularly on the environmentally sustainable multicomponent reactions (MCR) involving the use of Ionic Liquids (ILs) as a catalyst-solvent system.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


