RESEARCH ARTICLE
In Vitro and In Vivo Evaluation of the Protective Potential of Moringa oleifera Against Dietary Acrylamide-induced Toxicity
Lubna Rifai1, Mariam Mohammad2, Karim Raafat3, Fatima A Saleh2, *
Article Information
Identifiers and Pagination:
Year: 2020Volume: 14
First Page: 26
Last Page: 34
Publisher ID: TOMCJ-14-26
DOI: 10.2174/1874104502014010026
Article History:
Received Date: 24/2/2020Revision Received Date: 11/5/2020
Acceptance Date: 12/5/2020
Electronic publication date: 30/07/2020
Collection year: 2020

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
A c rylamide (AA) in food is a public health concern that has attracted scientists’ attention worldwide.
Objective:
This study was carried out to investigate the efficiency of Moringa oleifera (M. olifera) leaves in the reduction of AA in French fries in vitro and its hepato-protective properties against AA-induced liver toxicity in vivo.
Materials and Methods:
Total phenolic, flavonoid, tannin contents and antioxidant potential of M. oleifera leaves were evaluated and the phenolic constituents characterized via HPLC. AA content was also monitored in French fries using LC-MS/MS. For in vivo assay, mice were treated with AA alone or in combination with M. oleifera (150 and 250 mg/kg IP).
Results:
Phytochemical screening showed that gallic acid, ellagic acid, epicatechin, and quercetin were the most abundant phenolic compounds identified. This work also demonstrated a nearly 37% reduction in AA when French fries were soaked in 1% M. oleifera. Moreover, AA-intoxicated mice resulted in a significant (P < 0.05) elevation in the liver enzyme alanine aminotransferase (ALT), which was restored when pre-treated with M. oleifera.
Conclusion:
This study proved that M. olifera could be effective in reducing AA levels in French fries and that treatment with M. oleifera extract can restore the hepatic damage in AA-intoxicated mice.
1. INTRODUCTION
For many years, people have been using heat to cook their food. Heating of food is important to ensure microbiological safety, nutritional quality and the desired sensory properties [1]. However, heating could cause the generation of less favourable substances, so-called thermal process contaminants [2, 3]. One such compound that has received much scientific interest in recent years is acrylamide (AA) [4]. AA is formed in food products derived from raw materials that are rich in carbohydrates and low in proteins, such as potatoes, when thermally processed at high temperatures (>120°C) [5, 6]. Potato (Solanum tuberosum) is a major agricultural crop consumed daily by millions of people around the world [7]. During frying, AA is formed as a result of the Maillard reaction, which is a non-enzymatic browning reaction between asparagines and reducing sugars found in potatoes [8].
Several factors affect the formation of AA in foods, including temperature and time of the heating process, the asparagines and reducing sugars contents as well as pH and moisture content [9]. Manipulation of these factors can regulate AA content in food. For instance, a simple measure of pre-soaking potatoes in water before frying can reduce the formation of AA as soaking decreases the glucose content in the potato strips and in turn reduces AA formation [8]. Blanching in hot or warm water was also reported to attenuate AA formation in French fries not only by reducing the sugar levels of the raw potatoes, but also by decreasing the asparagines content [10].
Studies have shown that animal exposure to AA causes reproductive toxicity, genotoxicity, neurotoxicity and hepatic damage [11]. However, only neurotoxicity has been reported in human studies from occupational sources [12]. Because of the implications of AA-induced toxicities, investigators were interested in finding natural protective compounds that can reduce AA toxic effects. Studies of natural plants have highlighted the valuable content of numerous polyphenols, which can act as protective agents against AA toxicities. Becalski & colleagues (2002) have shown that AA concentrations were reduced in fried potato slices when rosemary herb, which has a powerful antioxidant activity, was added to the frying oil [13]. Moreover, Zhang and co-authors (2007) demonstrated that more than 70% of AA was reduced in potato crisps and French fries when dipped into bamboo-leaf extracts, rich in antioxidants [14].
Moringa oleifera (M. oleifera) belonging to the Moringaceae family, also known as “The Miracle Tree”, is a medium-sized tree that grows widely in many tropical and subtropical countries such as Asia, India, Africa, South and Central America [15]. Recently, this plant has been widely used throughout the world as a valuable food source because of its impressive range of medicinal uses and nutritional properties [16]. M. oleifera has also been found to exhibit antioxidant activity due to the high concentrations of antioxidants present in its leaves thus preventing oxidative stress related to many diseases [17].
Currently, many studies have proved the effectiveness of several natural compounds against the harmful effects of AA; however, there have been no reports on whether M. oleifera can have such a protective effect. Therefore, in light of many health-promoting benefits attributed to the consumption of M. oleifera, the present study focused on evaluating the phytochemical constituents and antioxidant activity of organic M. oleifera leaves and investigating its potential for reducing AA formation in French fries. Sensory analysis was also conducted in a double-blinded manner. Furthermore, the current study sought to demonstrate the protective effect of this plant against AA-induced hepatotoxicity in mice.
2. MATERIALS AND METHODS
2.1. Plant Material and Chemicals
Organic M. oleifera leaf powder was purchased from MicroIngredientsTM (US); whereas, Lebanese potato tubers and sunflower oil were collected from local commercial sources. Aluminum chloride salt (AlCl3), sodium carbonate anhydrous (NaCO3), absolute ethanol (EtOH, ≥ 99.8% purity), primary secondary amine (PSA) and C18 were obtained from Merck chemical company (Germany). Folin-Ciocalteu (FC) phenol reagent, 2,2- diphenyl-picrylhydrazyl (DPPH), sodium nitrite (NaNO2, ≥ 99.0% purity), gallic acid (C7H6O5), catechin reagents (C15H14O6), absolute methanol (MeOH), magnesium sulphate (MgSO4), and sodium chloride (NaCl) were purchased from Sigma-Aldrich (US). Besides, hydrochloric acid (HCL, 12N), sodium hydroxide (NaOH, 1M), acetonitrile (MeCN) and acrylamide (AA, 99%) standard were obtained from Fluka Company (Switzerland).
2.2. M. oleifera Extraction and Dry Matter Content
The dry matter content of the plant was determined by weighing the appropriate amount of the powder and drying it for 24 hours at 105°C. For extraction, the powdered plant leaf material (120 g/360 ml) was macerated with 80% EtOH for 24 hours at 4oC [18, 19]. The homogenate mixture was then filtered with the filtrate stored in the dark at 4oC prior to phytochemical analysis. For in vivo work, the extract was concentrated in an oven at 40oC for 48 hours. Then, 0.5 g of the obtained powder was dissolved in 12.5 ml normal saline to obtain a working solution (40mg/ml) which was stored at 4oC for further experiments.
2.3. In Vitro Experimental Study
2.3.1. Determination of Total Phenol Content
Total phenol content (TPC) in ethanolic M. oleifera leaf extract was determined by the Folin-Ciocalteau (FC) method using gallic acid as a standard [20]. Briefly, 0.2 ml of the extract was mixed with an FC reagent solution. After 5 min of incubation in the dark at room temperature, 0.8 ml of Na2CO3 (75mg/l) was added and then incubated for 10 min at 60°C. The assay mixture was subjected to a colorimetric measurement at 750nm by UV-VIS spectrophotometer (Gold S54T UV-VIS, China). A blank was prepared with distilled water. TPC in the extract was calculated using the equation: TPC= C*V/m (where C is the sample concentration from the calibration curve (mg/ml), V is the volume (ml) of the solvent used for the extraction, and m represents the weight (g) of the dried sample used) and expressed in mg of Gallic acid equivalence /g dry Dry matter (mg GAE/ g DM). The experiment was done in triplicates and the mean value ± standard deviation (SD) was presented.
2.3.2. Determination of Total Flavonoid Content
Total Flavonoid Content (TFC) in the M. oleifera leaf extract was determined using aluminum trichloride colorimetric method with some modifications and reported as catechin equivalents (CE) per gram of dried weight sample (mg CE/g DM) [21]. This method is based on the formation of a flavonoid-aluminum complex. Briefly, 1 ml of the extract was added into 4 ml of distilled water followed by 0.3 ml of NaNO2. After 5 min, 0.3 ml of AlCl3 was added to the mixture and at the 6th minute, 2 ml NaOH was poured and mixed. Absorbance was then measured at 510 nm by UV-VIS spectrophotometer. A blank was prepared with distilled water. The TFC of the extracts was calculated using the following equation: TFC = C*V/m.
2.3.3. Determination of Tannin Concentration
Total tannin content (g/l) was determined according to Ribérau-Gayonand and collaborators [22]. Total tannin assay is based on the heating process of tannins in an acidic medium leading to the formation of cyanidins. Two tubes were prepared, each containing 1 ml of the M. Oleifera extract sample, 0.5 ml of water and 1.5 ml of 12 N HCl. The first tube was mixed and heated in a water bath at 100°C for 30 min while the second was kept at room temperature for the same duration. Following rapid cooling, 0.25 ml of ethanol was added to the mixture and the resulting absorbance was recorded at 520 nm.
2.3.4. Evaluation of Radical Scavenging Activity
The DPPH radical was used in the current study to screen the radical scavenging activity of M. oleifera extract [23]. In short, 4 ml of 1 mM methanolic DPPH solution was added to 0.2 ml of M. oleifera leaf extract. The colorimetric changes from deep-violet to light-yellow after DPPH reduction can be quantified by the decrease in absorbance at 517 nm, after being incubated at room temperature for 30 min in darkness. Methanol was used as the blank. Absorbance of the DPPH radical without antioxidant (the control) was measured. The radical scavenging ability of the sample to scavenge the DPPH radical was evaluated using DPPH scavenging effect (inhibition %) = (Ac−As)×100/Ac, where Ac is the absorbance of the control and As is the absorbance of the sample.
2.3.5. High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) Analysis
Chromatographic analyses were carried out using a mobile phase that consisted of 1% formic acid in double-distilled water (57%) and methanol (43%). The flow rate was 0.7 ml/min at 40°C.
2.3.6. Evaluation of Acrylamide Content in French Fries
2.3.6.1. Sample Preparation
Potato tubers were washed, hand-peeled and cut into strips (1cm x 1cm x 3cm) with a French fries-shaped cutter and rinsed again for 1 min to eliminate starch material adhering to the surface before frying. Raw potato strips were then randomly allocated into separate groups and pre-treated with different solutions to investigate their impact on AA formation. A previously weighed-out 100-gram of potato strips were soaked for 30 min in tap water alone (1L) and tap water with added 1% and 5% M. oleifera leaf powder. A control sample (without any pre-treatment) was also included. Potato strips from each group were then drained and placed separately into a frying basket of an electrical fryer machine and submerged into hot sunflower oil (2.5 L) at 180 ± 5°C for exactly 4.5 min. The fries were then drained for 15 seconds, blotted between two layers of paper towels and cooled at room temperature for about 30 min. Finally, the samples were dried in an oven at 50oC for 48 hours and blended down by an electrical blender until a fine powder was produced. The homogenized powdered French fries were then stored at – 20oC for further analysis.
2.3.6.2. Acrylamide Quantification by LC-MS/MS
AA contents (µg/kg) in potato samples were extracted using QuEChERS method [24]. QuEChERS works by using water and acetonitrile to facilitate AA extraction from French fries. To extract AA, 10 ml of water was added to 5 g of thoroughly homogenized samples, vortexed briefly followed by the addition of 10 ml of MeCN. Then 4 g of anhydrous MgSO4 and 1 g of NaCl were added. Immediately after, the tubes were sealed and shaken vigorously for 1 min to prevent the formation of crystalline agglomerates and to ensure sufficient solvent interaction with the entire sample followed by centrifugation for 10 min at 5000 rpm. 1 ml of the MeCN extract was then transferred to a microcentrifuge tube containing 150 mg of PSA, 50 mg end-capped C18 and 150 mg of anhydrous MgSO4 and mixed for 30 seconds. The dispersive solid-phase extraction (SPE) step using PSA is crucial in order to remove any residual carbohydrates or proteins that may interfere with the chromatography analysis. PSA and C18 eliminate the need for hexane defatting and result in clean extracts [25]. Finally, the mixture was centrifuged for 1 min at 5000 rpm and 500 µl of the supernatant was transferred into an autosampler vial for LC-MS/MS analysis.
2.4. In Vivo Experimental Study
2.4.1. Experimental Animals
This study was carried out using Male Swiss-Webster mice of 12-16 weeks with initial weight 22-30g, provided by the animal house of Beirut Arab University (BAU) and kept in stainless steel cages under standard laboratory conditions of uniform humidity (50-60%) and temperature (21-23oC) with 12/12 hour light/dark cycle. They were allowed free access to water and standard laboratory pellet diet.
2.4.2. Experimental Design
Mice were randomly divided into 4 groups of 4 animals each and were treated with the particular treatment under consideration as follows; Group I is the control (CTRL) group, Group II is the acrylamide only (AA) group, Group III is the
| Group | n | Treatment | Abbreviation of group | Description |
|---|---|---|---|---|
| I | 4 | saline | CTRL | Normal mice: Vehicle given to mice IP |
| II | 4 | AA (50 mg/kg) | AA | Acrylamide given to mice IP |
| III | 4 | AA 50 mg/kg + M. oleifera ethanolic extract 150mg/kg | AA+ MO low | Acrylamide and M. olifera low dose given to mice IP |
| IV | 4 | AA 50 mg/kg + M. oleifera ethanolic extract 250 mg/kg | AA+ MO high | Acrylamide and M. oleifera high dose given to mice IP |
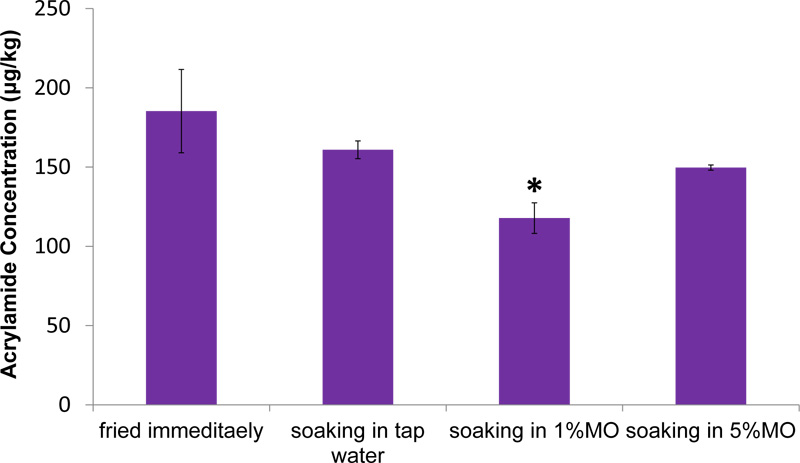 |
Fig. (1). Timeline of in vivo experiments. |
AA plus low dose M. oleifera (AA+MLlow) group and group IV is the AA plus high dose M. oleifera (AA+MHhigh) group (Table 1). The M. oleifera extracts at low and high doses (150 and 250 mg/kg, IP) were administered daily for twelve days to Group III and IV respectively, whereas, AA (50 mg/kg IP) was administered on the 13th day for 4 consecutive days as shown in Fig. (1) concomitantly with M. Olifera injections. The AA treated group (Group II) received normal saline in place of M. oleifera extract and the CTRL group received saline only, with the same frequency and duration of treatment of the other groups (Table 1). The injections were given at the same time daily and animal body weights were monitored. At the end of the experiment, animals were sacrificed and serum was collected for subsequent investigation.
2.5. Statistical Analysis
SPSS version 24 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. For sensory analysis and acrylamide content in French fries, results were analyzed by one-way analysis of variance (ANOVA). Weight and liver test data were analyzed using two way and one way ANOVA, respectively. The analysis was followed by a Post hoc test to compare various groups with each other. Results were expressed as mean ± SD. Differences were considered statistically significant when P < 0.05. The Bonferroni correction was used to adjust confidence intervals.
3. RESULTS
3.1. Phytochemical Analysis of M. Oleifera Leaf Extract
M. oleifera leaf extract was subjected to phytochemical analysis for TPC, TFC and tannins as well as antioxidant activity by DPPH radical scavenging method. TPC determined following a modified Folin-Ciocalteu method was 519.87 ± 61.32 mg GAE/g dry matter. The amount of flavonoids measured was 950 ± 45 mg CE/g dry matter; whereas, the percentage of total tannin content of the M. oleifera leaf extract was 115.78 ± 4.10 mg/l as shown in Table 2 M. Oleifera also exhibited radical scavenging activity of 74.14 %.
3.2. HPLC Profile of M. Oleifera Leaf Ethanol Extract
Phenolic compounds in M. Oleifera ethanol extract were identified and quantified by HPLC as shown in Table 3. HPLC analysis showed the presence of phenolic compounds such as (1) gallic acid (30.7%), (2) catechin (4.13%), (3) chlorogenic acid (2.0%), (4) ellagic acid (30.6%), (5) epi-catechin (9.5%), (6) rutin (5.1%), (7) quercitrin (1.9%), (8) iso-quercitrin (4.8%), (9) quercetin (5.3%), and (10) Kaempferol (4.3%).
| Parameter (unit) | Value ± SD |
|---|---|
| Total phenolic content (gallic acid equivalent) (mg/g) | 519.87 ± 61.32 |
| Total Flavonoid content (catechin equivalent) (mg/g) | 950 ± 45 |
| Total tannins (mg/l) | 115.78 ± 4.10 |
| DPPH scavenging effect (%) | 74.14 ± 1.31 |
| Retention time Rt (min.) | Constituent | (%) |
|---|---|---|
| 4.7 | Gallic acid | 30.7 |
| 5.4 | catechin | 4.13 |
| 7.05 | Chlorogenic acid | 2.0 |
| 8.5 | Ellagic acid | 30.6 |
| 10.6 | epicatechin | 9.5 |
| 12.7 | rutin | 5.1 |
| 15.1 | quercitrin | 1.9 |
| 30.8 | Iso-quercitrin | 4.8 |
| 41.4 | quercetin | 5.3 |
| 49.3 | kaempferol | 4.3 |
3.3. Acrylamide Content in French Fries
Analysis of AA content in French fries showed that soaking potato strips in water reduced AA formation; however, the reduction was not significant (P > 0.05) compared to the control group which is fried immediately. Interestingly, the addition of M. oleifera to the soaking water reduced AA formation; however the reduction was only significant (P < 0.05) with the application of 1% M. Oleifera (Fig. 2).
3.4. Sensory Analysis
The sensory panel evaluated French fries based on several descriptors linked to appearance, flavor, texture and overall acceptability. The effects of different pre-treatments (1% Vitamin C, 1% M. oleifera and 5% M. oleifera) on sensory properties of French fries are presented in Fig. (3). Potatoes soaked in vitamin C had significant differences in flavor and overall acceptability compared to the control groups (P= 0.001), and 1% M. oleifera (P = 0.002) and 5% M. oleifera (P= 0.011) treated groups. However, the differences in appearance and texture were not significant (P > 0.05) compared to control and M. oleifera treated groups. The appearance, texture, flavor and overall acceptability of the French fries soaked in M. oleifera solutions were not significantly different compared with the control group (P > 0.05) (Fig. 3).
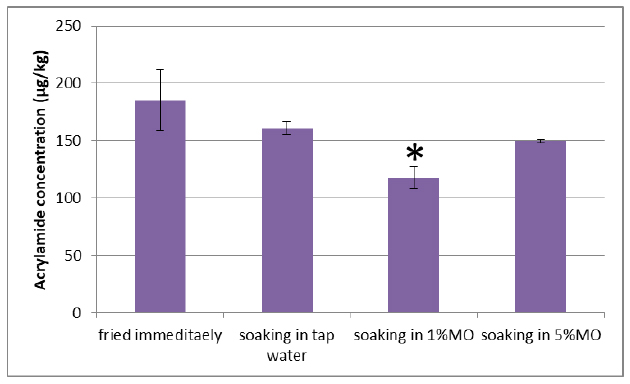 |
Fig. (2). Effect of various pre-treatments on acrylamide content in French fries *P < 0.05 indicates significance compared to control samples. |
 |
Fig. (3). Spider web chart of sensory analysis of different treatments on French fries. FI: Fried immediately, MO: M. Oleifera. *P < 0.05 indicates significance compared to control samples. |
3.5. Effect of M. oleifera Leaf Extract on Acrylamide-intoxicated Mice
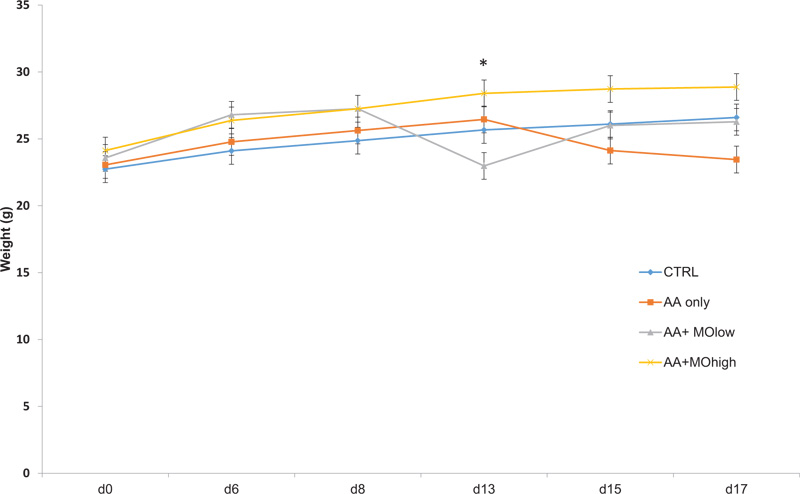
3.5.1. Bodyweight Changes
Fig. (4) presents bodyweight changes in mice groups as per response to different treatments. Mice injected with AA demonstrated loss in bodyweight compared to control untreated mice. Nevertheless, the administration of high dose M. oleifera (250 mg/kg) illustrated a significant increase (P < 0.05) in bodyweight compared to control mice (Fig. 4).
| Group | Treatments | n | Dose | Enzyme Activity | |
| ALT | AST | ||||
| CNTRL | Saline | 4 | - | 24.64+14.97 | 24.64+14.97 |
| AA only | Acrylamide | 4 | 50/mg/kg | 95.46*+1.03 | 32.04+3.55 |
| AA+MOlow | Acrylamide+low conc.of M.Olifera | 4 | 50/mg/kg+150/mg/kg | 34.20*+1.05 | 20.35+3.85 |
| AA+MOhigh | Acrylamide+high conc.of M.Olifera | 4 | 50/mg/kg+250/mg/kg | 62.67*+7.05 | 25.66+1.75 |
3.5.2. Liver Function Test
As shown in Table 4, AA administration significantly (P < 0.05) elevated serum ALT enzyme activity, which indicates liver damage. However, pre-treatment of mice with low and high doses of M. oleifera improved liver function enzymes, with significant (P < 0.05) reduction in ALT activity compared to control.
4. DISCUSSION
Many efforts have been made to reduce AA in the diet and to ameliorate its related toxicity in the body by the use of natural antioxidants. The present study was carried out to evaluate the antiradical activity of M. oleifera and to assess its ability to reduce AA formation in French fries. Additionally, as a powerful phenolic natural agent, the study sought to evaluate the possible protective effect of M. oleifera against AA-induced hepatotoxicity in vivo.
As reported in previous studies, this study demonstrated that M.oleifera is rich in various phytochemicals, mainly gallic and ellagic acid [17, 26]. This was in concurrence with other studies showing that gallic acid was among the most abundant phenolics in leaf extract of M. oleifera [27, 28]. M. oleifera also exhibited radical scavenging activity of 74%, which was similar to previously reported result by Falowo et al. (2017), whose investigation showed 75.9% inhibition of DPPH radicals by ethanolic extract of M. oleifera leaves [29].
AA concentration in French fries was also tested prior to and after treatment. Our findings revealed that soaking in water for 30 min was able to reduce AA levels in French fries, which was in accordance with other investigators who demonstrated reductions in AA formation when potato slices were soaked in water due to the decrease in the glucose content of the potato strips before frying [8, 9]. On the other side, soaking potato strips in water containing M. oleifera (1%), significantly reduced AA formation in French fries when compared to the control group (fried immediately). This can be explained by the fact that M. oleifera is rich in phenolic compounds. Several studies have reported how high levels of phenolic compounds may mitigate AA formation [30]. Ismial and colleagues (2013) demonstrated that soaking potato strips in phenolic solutions like ferulic acid, protocatechoic acid, caffeic acid, catechin and gallic acid for 60 min resulted in significant reductions in AA content in fried potatoes [30]. It is also worth noting that the organoleptic features of French fries were not affected by the M. oleifera treatment.
Besides, the present study determined that AA administration in vivo decreased bodyweight which is in agreement with other findings [31]. Mahmood et al. (2015) proposed that this decrease in bodyweight could result from the breakdown of tissue and blood cells in the liver and kidney by AA in rats [31]. However, M. oleifera treatment seems to significantly enhance bodyweight which was in accordance with Adeyemi and collaborators who reported improvements in bodyweight of mice when treated with M. oleifera after Nickel exposure [32]. This increase in the bodyweight of the group pre-treated with M. oleifera indicates its high nutritional values.
In order to confirm the AA-induced liver toxicity, liver enzymes (ALT and AST) activities were determined. AA intoxication showed hepatotoxicity manifested in a significant elevation in serum ALT which is in harmony with other studies [33-35]. However, serum AST level was not significantly different from that of the control group. Although both ALT and AST levels are usually elevated when the liver is intoxicated, ALT is more specific in detecting liver damage as a result of drugs or other substances that are toxic to the liver and, in some cases, may be the only one to be significantly increased [36-38]. This comes in line with Kim et al. (2008) who reported that the serum ALT activity is a reliable and sensitive marker of liver disease and is more commonly elevated than AST [39]. Furthermore, while increased AST levels are indicative of a tissue injury, it is not specific to the liver [40]. On contrary, any elevation of ALT is a direct indication of a liver injury [40]. Pretreatments of mice with M. oleifera extract were able to afford protection against AA-induced liver damage by significantly lowering the elevated ALT levels. This reduction might be a result of the regulation of liver cell membrane integrity by M. oleifera due to its antioxidant and free radical scavenging properties [32]. Herein, there is a clear demonstration of the hepato-protective effects of M. oleifera against Acrylamide-induced toxicity.
CONCLUSION
To the best of our knowledge, this study is the first to investigate the potential of M. oleifera in reducing AA formation in French fries as well as its hepato-protective effect against AA toxicity in vivo . Our findings show that the addition of M. oleifera presents an attractive and promising strategy to reduce AA formation in potato-based products while also retaining reasonable sensory attributes. M. oleifera can also be useful as a liver-protective natural agent especially when added to starch-containing foods before frying. The approach described here could be considered as a pioneer finding of a simple and natural way to reduce the amounts of AA in potato-based foods and its toxic effects, and thus can be an attractive candidate for future implementation in the food industry.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animals were treated according to ethical standards and the guidelines of the Ministry of Higher Education and Legislation of animal experiments with the approval of BAU-Institutional Review Board, Beirut, Lebanon (2019H-0055-HS-R-0310).
HUMAN AND ANIMAL RIGHTS
Human participants were not included in this study. All animal procedures were carried out while abiding by the US National Research Council's “Guide for the Care and Use of Laboratory Animals”.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
I would like to thank Mrs. Lama Hanbali for laboratory assistance.