All published articles of this journal are available on ScienceDirect.
Anethum graveolens L. Alleviates Sperm Damage by Limiting Oxidative Stress and Insulin Resistance in Diabetic Rats
Abstract
Background:
It has been reported that diabetes is associated with sperm damage and infertility.
Objective:
The purpose of this experiment was to survey the effect of Anethum graveolens L. (Dill) powder on sperm profiles, oxidative stress, insulin resistance, and histological changes in male diabetic rats.
Methods:
Male rats were randomly divided into 6 groups (n=7); group 1: normal rats, 2: normal rats + 100mg/kg Dill, 3: normal rats + 300mg/kg Dill, 4: diabetic rats, 5: diabetic rats + 100mg/kg Dill, and 6: diabetic rats + 300mg/kg Dill. After 2 months of treatments, the sperm profile, anti-oxidant activity, superoxide dismutase (SOD) activity, and malondialdehyde were measured. The histopathology of testis was evaluated. Hormonal changes and tumor necrosis factor-α (TNF-α) levels were measured by ELISA.
Results:
Total anti-oxidant and SOD activity in diabetic rats significantly decreased, while MDA concentration was significantly increased in the testis and pancreas of diabetic rats compared with control. However, the use of Dill significantly normalized these profiles. The treatment of diabetic rats with Dill changed the sperm parameters. The levels of testosterone, FSH, and LH in diabetic rats were significantly reduced, but the treatment with Dill did not alter the level of these hormones. Dill also significantly normalized testis morphological changes, insulin resistance, and inflammation.
Conclusion:
The use of Dill normalized oxidative stress, inflammation, and insulin resistance in diabetic rats that correlated with sperm profile and testis histological changes. The treatment of diabetic rat models with Dill did not show harmful effects on sperm profiles.
1. INTRODUCTION
Diabetes is a metabolic disorder that is related to a disturbance in carbohydrate, lipid, and protein metabolisms [1]. Obesity, inactivity, and consumption of high calorie diet significantly raise the prevalence of type 2 diabetes (T2D) worldwide. In this respect, the World Health Organization (WHO) reported that the prevalence of this disorder would reach 552 million by the year 2030 [2]. In diabetes, increasing inflammation and oxidative stress lead to dysfunction of pancreatic β-cells and a decline in insulin secretion [3]. Furthermore, inflammation and oxidative stress are related to all diabetic complications [4].
It has been reported that high free radicals and cytokines levels have potentially harmful effects on male fertility by inducing damages in lipids, DNA, testicular cells, and spermatozoa structure [5]. Consequently, reducing free radicals and inflammatory factors has a central role in the management of blood glucose, insulin levels, and diabetes complications [3, 6, 7]. In spite of various hypoglycemic medicines, patients tend to use herbal medicine for the treatment of diabetes due to low adverse effects and low cost. About 300 hypoglycemic and anti-oxidant herbal medicines have been recognized for diabetes management [8]. One of them is Dill (Anethum graveolens L.), which is a native plant of Asia and Europe. Interestingly, 5000 years ago, this plant was used by Egypt and Roman pharmacologists. Greeks covered their heads with this plant for the treatment of sleep disorders [9]. Many studies have shown useful effects of Dill on in vitro and in vivo experiments [9]. Different pharmacological effects of Dill, such as anti-diabetic, anti-secretory, and anti-ulcer activities, anti-hyperlipidemic, anti-hypercholesterolemic, anti-oxidant, anti-spasmodic, anti-microbial, and anti-inflammation have been established [10-14]. Some flavonoids isolated from Dill have potential anti-oxidant effects and could counteract with reactive oxygen species (ROS) and therefore, are capable of preventing diabetic complications [11]. Previous phytochemical studies, along with other reports indicated that Dill is a rich source of phenolic components, saponins, tannin, and flavonoids [10-14]. The aim of this study was to evaluate the effects of Dill consumption in testicular and pancreatic oxidative stress, insulin resistance, sperm profile, and hormonal changes in streptozotocin-induced diabetic animals. It was postulated that Dill administration could reduce insulin resistance and oxidative stress in the pancreas and testes of diabetic rats and prevent the harmful effects of diabetes on fertility.
2. METHODS AND MATERIALS
2.1. Animals
In this experiment, 42 adult male Wistar rats weighing about 220 g were adapted to the laboratory environment for one week and randomly divided to 6 groups of 7 rats as: group 1: normal rats, group 2: normal rats + 100mg/kg Dill, group 3: normal rats + 300mg/kg Dill, group 4: diabetic rats, group 5: diabetic rats + 100mg/kg Dill, group 6: diabetic rats + 300mg/kg Dill.
2.2. Induction of Diabetes
T2D was induced in overnight fasted rats using intraperitoneal (IP) injection of streptozotocin (STZ in citrate buffer pH 4.5) at the dose of 60 mg/kg followed by the injection of nicotinamide at the dose of 110 mg/kg after 15 min, to prevent up to 40% β-cells from STZ toxicity and to induce type-2 diabetic models instead of type-1 diabetes [15]. Blood glucose was measured after 72h and the levels more than 250 mg/dL were considered diabetic [16].
2.3. Experimental Design
The rats were housed in an animal house with good ventilation and a period of 12-h light/12-h dark cycle with ad libitum access to standard chow diet and water during the experiment.
Two months after the Dill treatment, rats were anesthetized, and blood was taken from the heart of the animals after overnight fasting. Serum samples were prepared and kept at -20°C for biochemical analysis. All treatments were performed daily for eight weeks to evaluate Dill effects.
2.4. Evaluation of Fertility Hormones
The serum levels of Follicle-stimulating Hormone (FSH), Luteinizing Hormone (LH), and testosterone was measured by the Enzyme-linked Immunosorbant Assay (ELISA) method using the rat commercial enzyme immunoassay kits, according to the manufacturer’s protocols (Zellbio, Germany) [17].
2.5. Sperm Profile
Epididymal spermatozoon was achieved by a puncture of cauda epididymidis with surgical blades in one ml Hams F'10. The sample was then thoroughly mixed to evaluate the progressive motility and viability by microscope within about 2 min of their isolation. The result was expressed as percentages. For determination of sperm count, the caudal epididymis sperm was diluted 10 times in normal saline. The spermatozoa were counted by hemocytometer, according to the previously published method [18]. The smear of the sperm suspension also was used for morphological evaluation and viability tests according to the previous method [18].
2.6. Anti-oxidant Activity
Total Anti-oxidant Capacity (TAC) in seminal plasma was determined according to Ferric reducing Anti-oxidant Power (FRAP) assay using small modifications [19]. Briefly, ferric tripyridyltriazine (Fe3+-TPTZ) complex was reduced to ferrous form (Fe2+-TPTZ) by the anti-oxidants in the sample at acidic pH [20, 21]. FeSO4, at the 100-1000 μmol/L concentration, was used as a standard and the results were calculated as μmol/L of FeSO4. The levels of Malondialdehyde (MDA) in seminal plasma and pancreas homogenate were determined by a colorimetric method based on Thiobarbituric Acid Reactive Substances (TBARS), as previously described [22, 23]. The SOD activity in seminal plasma and pancreas homogenate was determined using a commercial kit, according to the manufacturer's instructions (ZellBio, Germany) [24].
2.7. TNF-α Cytokine Assay
The inflammatory marker TNF-α was determined in serum by commercial kit using the ELISA method (BioLegend, UK) following the manufacturer's protocol [25].
2.8. Insulin Resistance
Fasting blood glucose and serum insulin were measured in order to calculate the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). Fasting blood glucose concentration was determined enzymatically by the glucose oxidase method using a commercial kit (Pars Azmun, Iran). Blood insulin levels were determined by the ELISA method using the kits purchased from Biocompare Co. with a detection limit of 28pg/mL. The HOMA-IR was calculated by the following formula: HOMA-IR = serum insulin (mmol/L)× blood glucose (mmol/L)/22.5.
2.9. Histopathology
One of the testes and a small part of the pancreas were removed, washed with PBS, and then fixed in 10% formalin solution. After the dehydration process, the testes and pancreas were blocked in paraffin, sections of 5µm were prepared, and stained with hematoxylin and eosin (H & E) and slides were examined under a light microscope [24, 26].
3. RESULTS
3.1. Hormonal Profile
The findings showed that luteinizing hormone (LH), follicle stimulating hormone (FSH), and testosterone significantly reduced in diabetic rats compared with the control group (p<0.05). Treatment with Dill had no significant effect on LH, FSH, and testosterone and estradiol levels of rats in the various treatment groups (Fig.1). However, treatment of normal rat with Dill significantly reduced testosterone levels as compared to untreated control animals.
3.2. Semen Analysis
Abnormal morphology, viability, motility, and count of sperms markedly reduced in diabetic animals in comparison with those of control rats (p<0.05); whereas, the administration of Dill significantly normalized these profiles compared to diabetic rats (p<0.05, Table 1).
3.3. Cytokine Assay
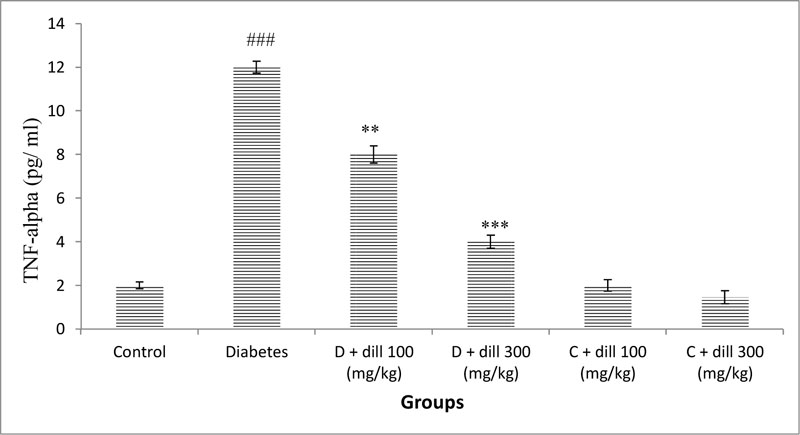
A significant rise in serum TNF-α was observed in the T2D rats compared with normal animals. Nevertheless, oral administration of Dill to T2D rats significantly decreased (p<0.05) TNF-α level when compared with untreated diabetic rats (Fig. 2).
3.4. Anti-oxidant Capacity and Malondialdehyde (MDA) Levels
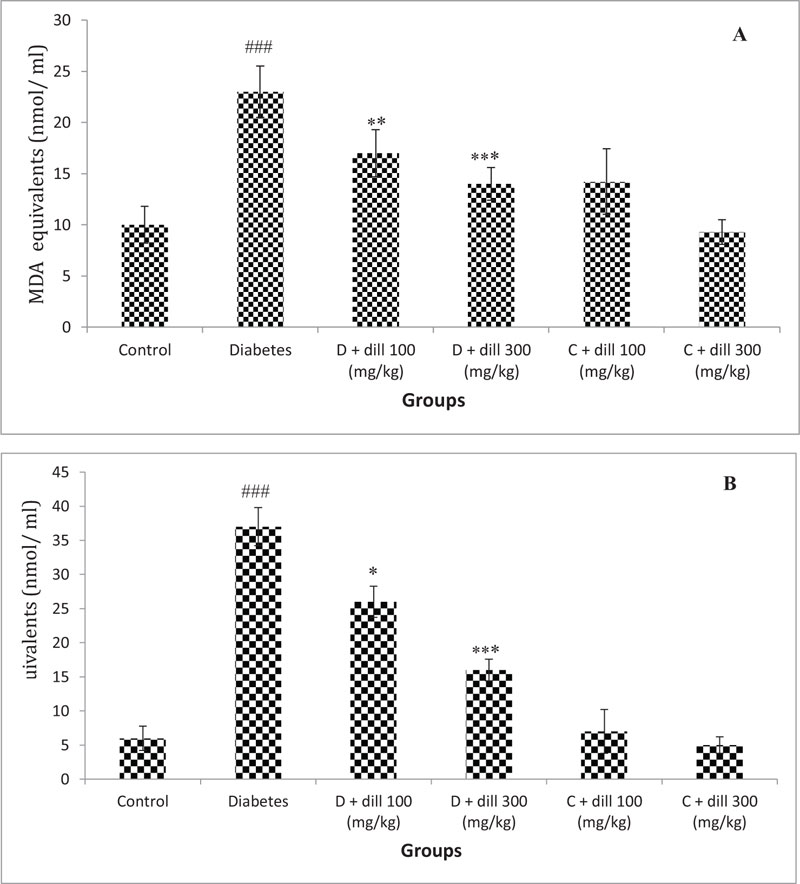
TAC in the seminal plasma and pancreas of treated groups were significantly reduced in T2D animals compared to healthy group (P<0.05). Treatment of T2D animals with Dill markedly increased TAC levels compared with untreated diabetic rats (P<0.05). Compared with normal rat, SOD activity was significantly lower in T2D rats (P<0.05). Treatment of diabetic rats with Dill increased the SOD activity when compared with diabetic group (Fig. 3).
T2D animals showed significantly higher MDA levels compared with the normal control group (p <0.05). After 2 months of treatment of T2D animals with Dill, the MDA levels significantly decreased compared to diabetic control (Fig. 4).
3.5. Serum Levels of Glucose, Insulin, and HOMA-IR
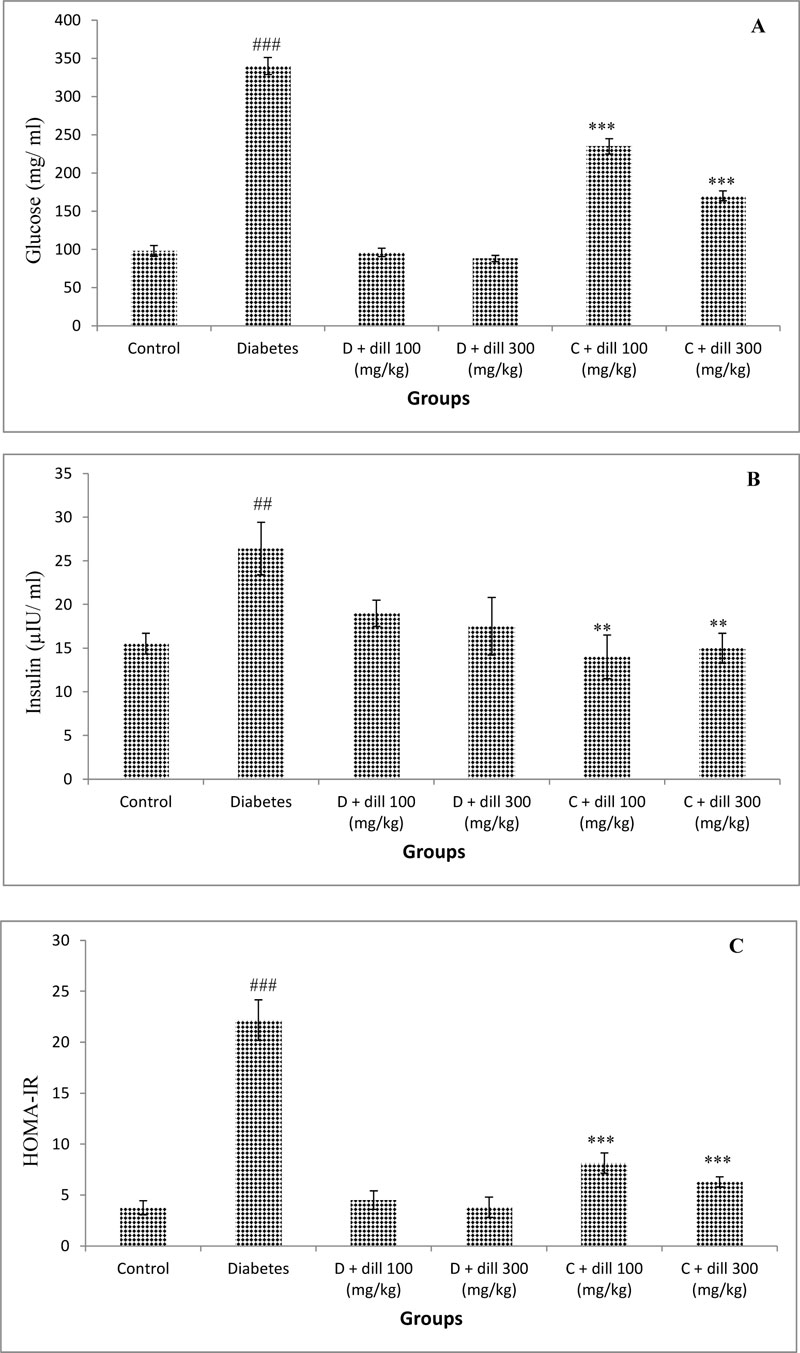
Serum insulin and glucose concentration were markedly higher in diabetic rats compared to normal rats (P<0.05). The treatment with Dill normalized insulin and glucose concentration. HOMA-IR was found markedly higher in the T2D animals comparing to normal rat; nevertheless, it was completely normalized after treatment of the rats with Dill (Fig. 5).
3.6. Histological Results
Fig. (4) displays histological changes in testis in different groups. Significant histological changes were observed in diabetic rats and in rats treated with Dill. Diabetic rats showed a significant decline in the diameter of the tubules and their epithelial heights concurrent with irregular seminiferous tubules, intertubular hemorrhage, and cytoplasmic vacuolization. These changes were significantly reduced in treated animals. Testicular tissues in treated animals exhibited marked raise in the spermatogenic activity. As illustrated in Fig. (4), most of the seminiferous tubules were repaired to the normal structure in Dill groups (Fig. 6).
4. DISCUSSION
It has been reported that diabetes is accompanied by augmented levels of apoptosis of germ cells in testes and disturbance of spermatogenesis [27, 28], which are associated with hormonal alterations, oxidative stress and hyperglycemia [29]. Diabetic subjects have reduced anti-oxidant capacity and/or raised oxidative damage in different tissues. High blood glucose in diabetes leads to oxidative stress because of increased ROS generation [6]. In this context, it is vital to find potential agents that scavenge free radicals and prevent the onset and/or development of diabetes or other diseases [30-34]. Dill has been established to have a variety of beneficial effects in diabetes [10]. In the recent experiments, Dill showed hypolipidemic and strong anti-oxidant properties [12, 14, 35]. Here Dill showed increased sperm quality and testis morphological change that was related to insulin sensitivity in diabetic animals. Herein, we studied the useful properties of Dill administration in T2D rats.
| Groups | Motility (%) | Viability (%) | Normal Morphology (%) | Sperm Count (x106sperm/mL) |
|---|---|---|---|---|
| Control | 68.0 ± 2.5 | 91.1 ± 4.5 | 86.5 ± 2.8 | 39.5 ± 8.0 |
| Diabetes | 53.8 ± 6.7* | 57.6 ± 4.0*** | 63.6 ± 3.7*** | 27.6 ± 5.1*** |
| Diabetes + Dill (100 mg/kg) | 55.5 ± 7.7 | 59.3 ± 6.4 | 87.0 ± 2.8 | 28.8 ± 7.4 |
| Diabetes + Dill (300 mg/kg) | 58.3 ± 5.3 | 59.3 ± 7.9 | 89.0 ± 3.3 | 29.8 ± 3.6 |
| Control + Dill (100 mg/kg) | 61.6 ± 5.4 | 69.5 ± 1.0## | 74.5 ± 9.4## | 35.6 ± 4.3 |
| Control + Dill (300 mg/kg) | 54.0 ± 1.0# | 72.6 ± 1.1## | 79.1 ± 6.3 | 40.8 ± 2.6 |
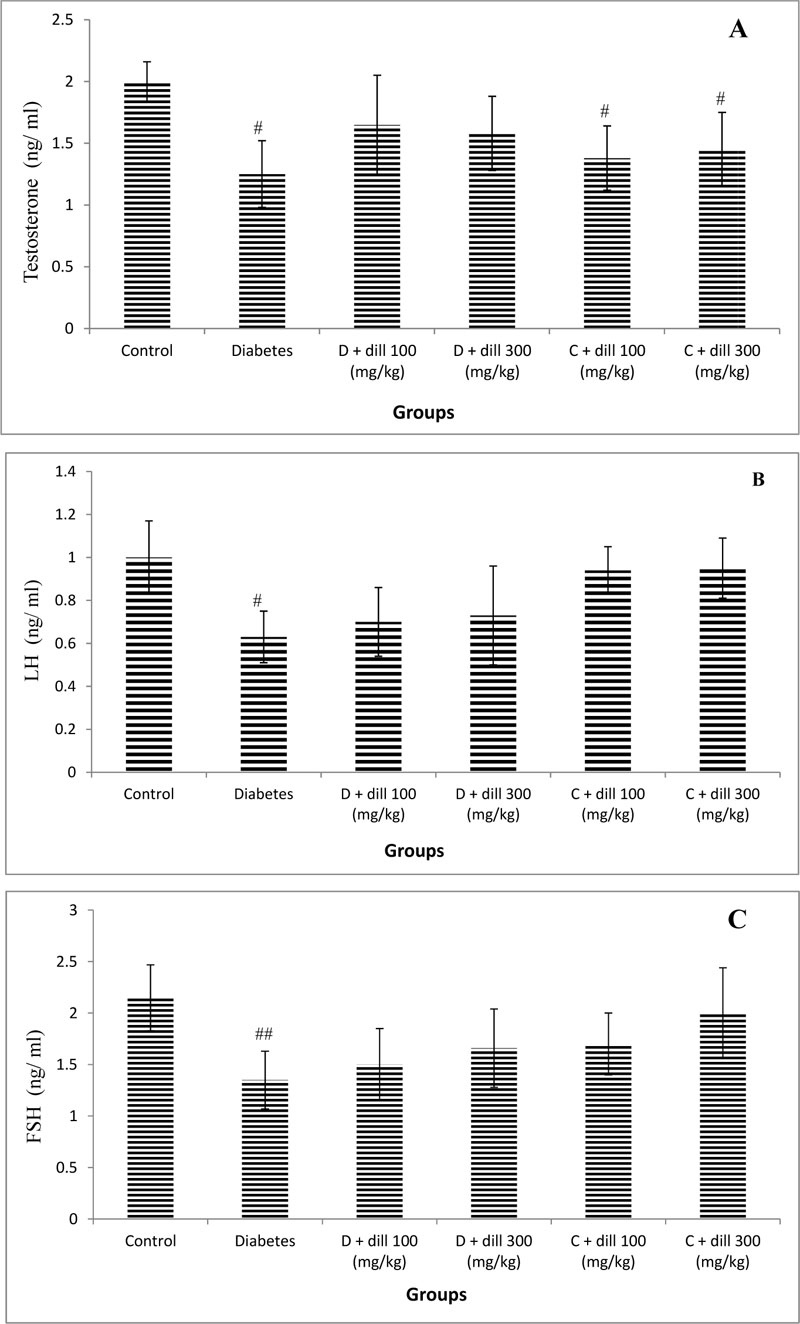

In this experiment, Dill treated animals showed reduced blood glucose levels and improved insulin resistance. In line with this study, various experiments established that Dill or its main constituents normalized hyperglycemia. Dill phytochemicals, such as quercetin, limonene, α-pinene, and isoliquiritigenin, ameliorate hyperglycemia by enhancing insulin action and anti-oxidant activity. Several experiments have reported that these agents declined blood glucose, triglycerides, cholesterol, HOMA-IR, inflammation, and oxidative stress [10-14]. Hence, it is suggested that an anti-hyperglycemic property of Dill is due to reducing insulin resistance and improving anti-oxidant capacity, mainly because of its high concentration of quercetin, limonene, α-pinene, and isoliquiritigenin. Various experiments showed that quercetin and limonene normalized β-cells structure and insulin secretion [10-14]. Quercetin content of Dill may attribute to protective and regenerative effects in β-cells. Quercetin administration also increases sperm motility and viability [36]. In Dill treated animals, the morphological changes of β-cells significantly normalized in diabetic rats. Dill also increased TAC, SOD activity and reduced MDA levels in the pancreas. In this study, Dill also ameliorated HOMA-IR in T2D rats. Notably, insulin resistance is of extreme importance in spermatogenesis since insulin controls the metabolic co-operation between testis and its need for normal testicular action [29].
The authors of this study showed that Dill increases total anti-oxidant in seminal plasma. High levels of testicular oxidative stress have harmful effects on male fertility [37]. Elevated oxidative stress in diabetes is accompanied by DNA damage causing infertility in animal models. Oxidative stress can also lead to damage to testicular lipids and proteins and especially spermatozoa. Previous studies showed that diabetes is associated with high oxidative stress-derived damage [6, 38], and consequently reduced testicular anti-oxidant capacity [38]; while, treatment of diabetic animals with Dill normalized anti-oxidant activity in testis tissue. In oxidative stress, the membrane lipids are oxidized and MDA (the end-product of lipid peroxidation) is increased that is determined by the TBARS assay [39]. MDA formation has vigorous harmful effects on male fertility which is caused by oxidative stress in diabetes. In this experiment, testicular MDA levels significantly increased in T2D animals; while, treatment with Dill markedly reduced MDA in T2D animals to their normal levels. SOD is known as a main anti-oxidant defense in the different tissue and its activity in seminal plasma correlates with sperm quality [40]. In this study, SOD activity significantly increased in the testis and pancreas of Dill treated group. Furthermore, sperm count, sperm viability, and motility significantly reduced in diabetic animals. Whereas, these factors were normalized in T2D animals treated with Dill. A large number of studies have established that sperm quality is significantly reduced in diabetes in comparison with controls [27]. This plant ameliorates sperm count, viability, and motility chiefly by inhibiting oxidative stress and insulin resistance. Monsefi et al. [41] reported that administration of the aqueous and ethanol extracts of Dill seed in normal Wistar rats (0.5 and 5 g/kg) could not change sperm count, sperm motility or testosterone levels. However, in this experiment, dill tablets were used to treat diabetic animals, which contains dill (68%), Cichorium intybus (5%), Fumaria parviflora (5%), and Citrus aurantifolia (4%) [42]. The results of Iamsaard et al. [43] showed that Dill extract increased protein phosphorylation in testis, proposing that Dill may motivate tyrosine phosphorylation of testicular proteins involved in spermatogenesis. They also showed that Dill extract has no effects on sperm physiology, particularly acrosome exocytosis, indicating that Dill is harmless to sperm profile. In this study, the levels of testosterone, LH, and FSH in diabetic animals significantly reduced compared with the normal control group. However, these hormonal changes in Dill treated groups were not statistically significant. On the other hand, testosterone levels significantly reduced in control groups. In diabetic animals, a declined pituitary response to gonadotropin-releasing hormone (GnRH) has been reported in diabetes [44]. Dill reduced serum TNF-α level in diabetic animals. TNF-α has a main role in the impairment of the insulin signaling cascade and leads to insulin resistance [45]. This marker also significantly reduced sperm function [46].
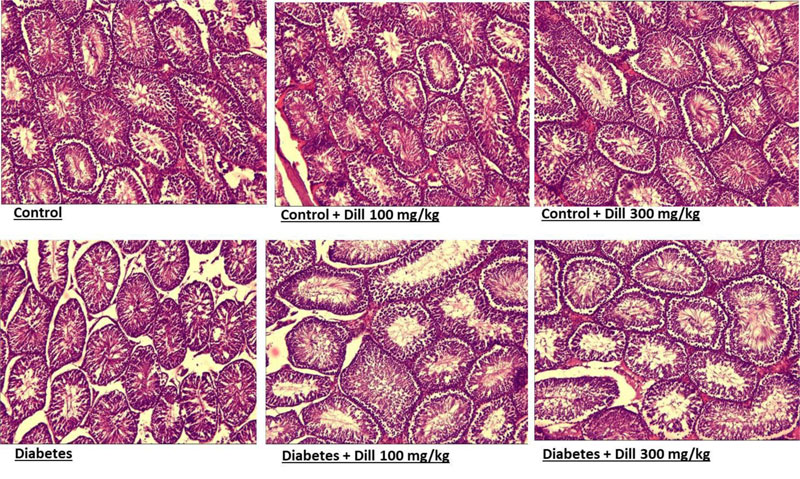
Diabetic animal models showed apoptosis in the testicular germ cell, abnormal spermatozoa, and low testosterone concentration [27]. In this study, testis tissue of diabetic animals showed irregular seminiferous tubules, intertubular hemorrhage, maturation arrest, and giant cell formation, and many of the spermatogonia displayed cytoplasmic vacuolization. In diabetic rats, the spermatogenic cells are also degenerated and exfoliated in the lumen of the tubules. These changes observed in diabetic rats were significantly normalized after 2 months of treatment with Dill. The augmented anti-oxidant capacity and reduced total oxidant and MDA levels were observed; also, unpleasant morphological changes were ameliorated in treated groups. In fact, it can be concluded that the increased oxidative environment in the testis of diabetic rats causes cellular damage.
CONCLUSION
Dill alleviated oxidative stress, insulin resistance, and improved sperm profile in diabetic rats. Therefore, Dill, which is used as a hypolipidemic agent, may show beneficial effects on sperm profile by reducing inflammatory markers, scavenging free radicals, and mitigating insulin resistance.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experiments were carried out according to the animal ethical committee of Hamadan University of Medical Sciences, Hamadan, Iran (ethical code: IR. UMSHA.REC.1394.578). All process of this experiment was done in accordance with the principles expressed in the Declaration of Helsinki.
HUMAN AND ANIMAL RIGHTS
Humans did not participate in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This work was supported by a Hamadan University of Medical Sciences grant (Project number: 9503251543).


