RESEARCH ARTICLE
Antitumor Activity In Vitro Provided by N-Alkyl-Nitroimidazole Compounds
Janny A. Villa-Pulgarin1, Constain H. Salamanca2, Jose Oñate-Garzón3, Ruben E Varela-M3, *
Article Information
Identifiers and Pagination:
Year: 2020Volume: 14
First Page: 45
Last Page: 48
Publisher ID: TOMCJ-14-45
DOI: 10.2174/1874104502014010045
Article History:
Received Date: 29/03/2020Revision Received Date: 05/06/2020
Acceptance Date: 22/06/2020
Electronic publication date: 30/07/2020
Collection year: 2020

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Cancer is one of the most common diseases in the world, with over 18 million new cases estimated in 2018. Many of the drugs used for cancer can have significant adverse effects and variable effectiveness. Nitroimidazoles are prodrugs that usually have shown antimicrobial activity specifically antiparasitic. However, its antitumor activity in vitro has barely been explored.
Objective:
The aim of this study is to determine the influence of the length of the substituted N-alkyl chain in the imidazole ring on the antitumor activity in vitro.
Methods:
Four nitroimidazoles were obtained by chemical synthesis varying the length of the substituted N-alkyl chain from methyl to butyl. The antitumor activity of N-alkyl-nitroimidazoles was evaluated by MTT assay employing two tumor cell lines (MDA-MB231 and A549).
Results:
In this study, it was reported that N-alkyl nitroimidazoles exhibited an LC50 as low as 16.7 µM in breast tumor cells MDA-MB231 while in normal Vero kidney cells, the LC50 was around 30 µM. It was also reported that the length of the substituted N-Alkyl chain in the imidazole ring affects the antitumoral activity in A549 lung cells.
Conclusion:
Increasing the length of the substituted N-Alkyl chain in the imidazole ring decreased the antitumor activity against only A549 cancer cells. N-alkyl nitroimidazoles exhibited considerable selectivity towards tumor cell lines.
1. INTRODUCTION
Cancer is considered a public health problem worldwide and in Colombia. According to the International Agency for Research on Cancer, it is estimated that there were 18.1 million new cancer cases and 9.6 million cancer deaths in 2018 [1,2]. The most commonly diagnosed cancer is lung cancer in men and breast cancer in women [3]. Due to the high number ofcases that occur, the large number of adverse effects and the low effectiveness that is achieved in many of the treatments, it is of great importance for many organizations to investigate new alternatives for their treatment. The current treatment modalities are surgical resection, radiation therapy, hormone therapy, anti-hormone therapy, combined therapy as chemotherapy and radiation therapy, where the most commonly used chemotherapeutic agents are cytotoxic agents for cancer cells [4]. Some of these agents correspond to the imidazole compounds, which are developed towards their application in medicinal chemistry due to their pharmacological properties, such as anticancer activity with high efficacy and low toxicity [5,6]. In this context, the imidazole ring containing two nitrogen atoms and desirable π-conjugated backbone can interfere with DNA synthesis, altering enzymes involved in DNA replication and the expression of genes associated with cancer cell growth such as tumor suppressor and cell cycle genes [7]. There are enough studies where imidazole ring is combined with heteroatom-containing groups in a single compound in order to generate compounds with improved anticancer activity [7]. Previous studies have reported that N-alkyl imidazoles display potent anticancer activity via inhibition heme oxygenase (HO-1 and HO-2), an enzyme related to certain types of cancers [8,9].
On the other hand, the length of the alkyl chain on the imidazole ring is relevant for antimicrobial activity. Khabnadideh et al., 2003 [10] showed that antibacterial activity of 1-alkylimidazoles against Gram-negative and Gram-positive bacteria increases, as the number of carbons in the alkyl chain rises up to nine and then decreases. However, the knowledge about the contributions of the number of carbons in the acyl chain on anticancer activity is reduced. N-alkyl-nitroimidazoles with different carbon numbers in the alkyl chain were synthesized and theoretically studied in previous research [11]. Herein, we describe the effect of the alkyl chain length substituted in the N-imidazole ring on the anticancer activity against cell lines A549 (Human lung carcinoma) and MDA-MB-231 (Human breast adenocarcinoma).
2. MATERIALS AND METHODS
The N-alkyl-nitroimidazoles used in this study were previously synthesized, characterized, cryopreserved (-20 ° C) and supplied by the laboratory of design and formulation of the Icesi University [11], which were used as received. The human cancer cell lines A549 (Human lung carcinoma) and MDA-MB-231 (Human breast adenocarcinoma) were obtained from the American Type Culture Collection (ATCC). The cells were cultured in culture flasks with DMEM (Gibco, Life Technologies Corporation), supplemented with 10% FBS and penicillin (100 U/mL) and streptomycin (100 µg/mL) and were kept in a humidified incubator containing 5% CO2 at 37°C.
2.1. Cell Growth Inhibition Assay
Cell proliferation and viability were assessed by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) assay, through MTT conversion into colored formazan product by metabolically active cells [12]. Cells (1,5 x 103 in 100 μL in 96-well flat-bottomed microliter plates) were incubated in DMEM culture medium containing 10% heat-inactivated FBS and were permitted to adhere for 12 h. After the culture medium was removed, 100 µL of different concentrations of nitroimidazoles (1, 5, 10, 25, 50 and 100 µM), prepared in DMEM, were added to each well. Following 48 h of incubation at 37ºC in a humidified atmosphere of air/CO2 (19/1), the MTT assay was performed. Measurements were done in triplicate. Absorbance was measured using an IMARKTM reader with a reference filter at 630 nm and a reading filter at 570 nm. The fraction of viable cells was calculated relative to control cells based on the following equation: (A1/A2) 100%, where A1 and A2 represent the absorbance of the sample and control groups, respectively. Each determination was performed in triplicate.
3. RESULTS
The in vitro antitumor activity for compounds was determined by the measurement of their cytostatic and cytotoxic properties in human tumor cell lines by MTT assays. The human cell lines used were MDA-MB-231 (human breast adenocarcinoma) and A549 (human lung carcinoma). As shown in Table 1, both breast cancer cells and lung cancer cells are sensitive to the compounds used; however, lung cancer cells were more sensitive to N-methyl-nitroimidazole and N-ethyl-nitroimidazole. Furthermore, the length of the alkyl chain influenced on the antitumoral activity against only A549 cell lines. As can be seen, all the compounds showed less activity against normal cells, as are Vero cells. Doxorubicin used as a positive control rendered IC50 values in the order of 10−7–10−8 M (data not shown).
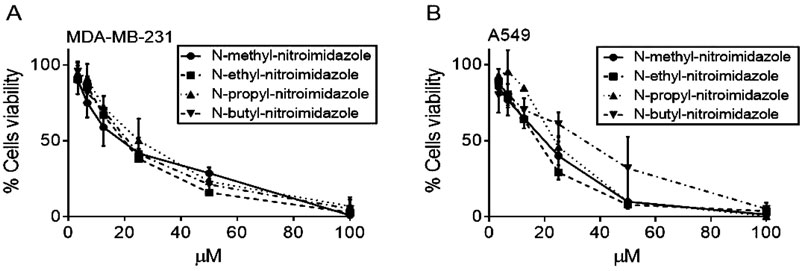
In order to determine which of N-alkyl-nitroimidazole compounds displayed a higher cytotoxic activity, cell viability was evaluated, through the incorporation MTT assay A concentration-response curve was observed between concentration and viability. A decrease in the viability of both cancer cell lines regarding the increase in the N-alkyl-nitroimidazole concentration was evaluated and is depicted in Fig. (1). The results showed that the four N-alkyl-nitroimidazole compounds described greater activity against breast cancer than against lung cancer, where the N-methyl-nitroimidazole and N-ethyl-nitroimidazole compounds corresponded to those with the highest cytotoxic activity. (Fig. 1).
4. DISCUSSION
Anticancer activity measured as the LC50 in cancer cell lines decreased as the length of the substituted N-alkyl chain increased, solely for the A549 cell line. Regarding the MDA-MB231 cell line, the anticancer activity did not vary considerably when the length of the alkyl chain increased, suggesting that the number of carbons in the substituted N-alkyl did not influence on the activity against this type of cell.
There are several studies where the antibacterial and antiparasitic activity of nitroimidazoles is described [13–18]. Al-Masoudi et al., reported that nitroimidazoles synthesized by a simple method exhibited some cytostatic activity against leukemia and melanoma cell lines [19]. In a previous study, nitroimidazole derivative of polypyridyl ruthenium complex was employed in order to evaluate the anticancer activity against breast cancer (4T1) and A549 cell lines and they reported that the tested cell lines were strongly inhibited with IC50 ranging from 1.5 to 18.8 µM [20], a range covering the LC50 values exhibited by N-methyl and N-ethyl-nitroimidazoles. In a study conducted by Kumar et al., 1H-1,2,3-Triazole Tethered Nitroimidazole−Isatin Conjugates showed IC50s of 54.25 and 26.12 μM against MCF-7 and MDA-MB-231 cell lines, respectively, when a butyl alkyl chain length as a spacer and a halogen-substituent on the isatin ring was placed [6]. LC50 values reported in our study are below those reported by Kumar et al. in MDA-MB-231 cell lines. Interestingly, Kumar et al., reported that the activity tends to increase with the increase in chain length in contrast with our results where activity decreased and was maintained with increasing chain length, against A549 and MDA-MB-231 cell lines, respectively. Furthermore, unlike our results, another study has reported that anticancer activity increases with the increase in the alkyl chain at imidazolium-based ionic liquids [21], suggesting an interesting and poorly reported finding that could be explored in subsequent studies.
Here, the results also suggest a high selectivity of N-alkyl-nitroimidazoles toward cancer cells. Potential targets can be provided from imidazole derivatives to understand such selectivity. Heme oxygenase, an enzyme involved in the heme group catabolism, has been associated with the proliferation of A549 cancer cell line [22] and has been identified as a selective target for functional groups such as azolyl moiety, phenyl groups, and alkyl linkages [23]. Another selective target of imidazole derivatives towards tumor cells is the cRAF-kinase protein, which is up-regulated in melanomas and alteration of this signaling protein would play a major role in melanoma progression [24,25]. DNA is also known as another anticancer action target for imidazole derivatives. Chen et al. [26] described that triaryl-substituted imidazole was a telomeric G-quadruplex ligand which alters telomere maintenance, a crucial event for the unlimited proliferative potential of cancer cells.
CONCLUSION
In conclusion, compounds with increased antitumor activity and low cytotoxic effect in normal cells, may become therapeutic candidates for the treatment of cancer. Here, N-alkyl nitroimidazoles showed a reduced LC50 in lung cancer cells A549 and breast cancer MDA-MB231, while in Vero kidney cells, the LC50 was almost double for the case of N-ethyl-nitroimidazole. Interestingly, only in lung tumor cells, the length of the N-alkyl chain of nitroimidazole is inversely proportional to the anticancer activity. Therefore, the pronounced selectivity of these compounds to tumor cells is a property that should be explored in future trials to contribute to the design of new drugs that can potentially be used in clinical practice.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.